This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Venipuncture pain is an uncomfortable suffering to the patient. It creates anxiety, fear and dissatisfaction. The ketoprofen transdermal patch is a proven treatment for musculoskeletal and arthritic pain. We planned this study to evaluate the efficacy of the ketoprofen patch to reduce venipuncture pain.
Methods
Two hundred adult patients, aged 18–60 years, of either sex, ASA grade I or II, were enrolled. Presuming that therapy would decrease venipuncture pain by 30%, a power calculation with α = 0.05 and β = 0.80 required enrollment of at least 24 patients into each group. However, 100 patients in each group were recruited. Group I (Control) received a placebo patch; Group II (Ketoprofen) received a 20 mg ketoprofen patch. A selected vein on the dorsum of the patient's non-dominant hand was cannulated with 18 g intravenous cannula 1 h after the application of the respective patch. Assessment of pain was done by a 10 cm visual analogue scale (VAS) of 0–10, where 0 depicts “no pain” and 10 is “the worst imaginable pain”. The venipuncture site was assessed for the presence of skin erythema, swelling and rashes at 12 h, 24 h and at the time of decannulation.
Results
Incidence of pain was 100% (94/94) in the control group as compared to 93% (85/91) in the ketoprofen group. The severity of the venipuncture pain was 6 (2) and 2 (2) for control and ketoprofen groups respectively (P < 0.05).
Conclusions
Application of a ketoprofen patch at the proposed site of venipuncture one hour before the attempt is effective and safe for attenuating venipuncture pain.
Go to :

Keywords: Erythema, Ketoprofen, Nonsteroidal anti-inflammatory drugs, Pain, Venipuncture, Visual analog scale
INTRODUCTION
Venipuncture pain is an uncomfortable suffering to the patient. This pain creates psychological problems like anxiety, fear, and dissatisfaction. Pathological side effects, like asystole following venipuncture, have been documented [
12]. Venipuncture pain attenuation may help to build trust between patient and health care personnel.
Considering analgesia as an interim part of providing anesthesia, several methods like an eutectic mixture of local anesthetic (EMLA), diclofenac patch, ibuprofen patch, diclofenac gel, and tricks like cough and Valsalva have been tried to avert venipuncture pain [
345678], but none of these methods deals with the problem perfectly, hence the search for a new drug or trick continues.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have an analgesic effect for acute as well as mild to moderate chronic pain [
9]. Ketoprofen is one example. It is a propionic acid derivative, with a plasma half life of 2–3 hrs. Like other NSAIDs, it has anti-inflammatory, anti-pyretic, and analgesic action, but additionally also stabilizes the lysosomes by inhibiting the lipoxygenase enzyme. All propionic acid derivatives are well absorbed orally and are highly bound to plasma proteins. They enter the brain, synovial fluid, and cross the placenta. They are metabolized in the liver by hydroxylation and glucuronide conjugation, and are excreted in the bile and urine [
10].
One of the most distressing symptoms of gastrointestinal intolerance of the NSAIDs can be avoided if the drug is topically applied [
31112]. It has been used in the form of a transdermal patch for the treatment of musculoskeletal and arthritic pain [
13]. Therefore, the idea to use a ketoprofen patch which might reduce venipuncture pain is appealing.
This double blind, randomized study was planned to determine the efficacy, tolerability, and acceptability of topical application of a 20 mg ketoprofen transdermal patch, to attenuate venipuncture pain.
Go to :

MATERIALS AND METHODS
After getting Institutional Review Board (IRB) approval (IEC code: 2013-49-IP-69), this prospective, randomized, double blind, placebo-controlled study was conducted from February 2014 to February 2015. It is registered at CTRI/2017/01/007694.
Following written and informed consent, 200 consecutive adult patients, aged 18–60 years, of either sex, ASA physical status I or II, and scheduled for elective gastrointestinal surgeries, were enrolled for this study.
Patients with skin allergies, a known allergy to any nonsteroidal anti-inflammatory drugs (NSAIDS), mental illness, pregnancy or taking any analgesic, or those unable to be cannulated in the first attempt were excluded from the study.
Presuming that therapy would decrease venipuncture pain by 30%, a power calculation with α = 0.05 and β = 0.80 required enrolment of at least 24 patients into each group. However, anticipating major dropouts and to observe local reactions, 100 patients in each group were recruited. 185 patients (92.5%) completed the study as 9 patients in group II and 6 patients in group I could not be cannulated in the first attempt, so these 15 patients (7.5%) were excluded from the study. A total of 185 patients were analysed from statistical point of view (
Fig. 1). Groups were comparable as regards to the demographic variables age, sex, height and weight (
Table 1).
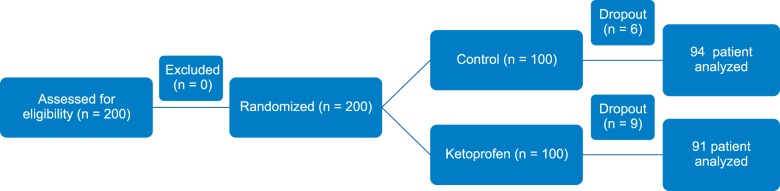 | Fig. 1Two hundred patients were eligible for the study. After randomization all of them were posted for the study. 185 patients (87%) completed the study. Only fifteen (7.5%) were excluded from the study. Out of them, 9 patients belonging to ketoprofen group and 6 patients of control group could not be cannulated in first attempt. Total 185 patients were analyzed from statistical point of view.
|
Table 1
Demographic Data


Patients were randomly allocated into one of the two groups, using a computer generated table of random numbers. Group I (Control) received a placebo patch; Group II (Ketoprofen) received a 20 mg ketoprofen transdermal patch (Artho-touch, Teikoku, Seiyaku Co., Ltd., Japan) each of 70 cm2 dimension. The vein on the dorsum of patient's non-dominant hand was selected as the proposed site of venous cannulation. Patches were applied at the proposed site one hour prior to the cannulation. Patients were kept unaware of the type of patch applied. A staff nurse, blinded to the patch type, participated in the application preoperatively.
Assessment of venipuncture pain was done by an anesthesia registrar (AR) who was kept blinded to the group allocation. Assessment of pain was done by a 10 cm visual analogue scale (VAS) of 0–10, where 0 stands for “no pain” and 10 stands for “worst imaginable pain”. Depending on the VAS, pain was graded as mild (VAS 1–3), moderate (VAS 4–6), or severe: (VAS 7–10). The incidence of venipuncture pain is derived from the number of patients with venipuncture pain of VAS ≥ 1 during venipuncture divided by the total number of patients initially at risk.
Venipuncture on the dorsum of the non-dominant hand was performed with an 18-gauge intravenous cannula (BD Venflon, 30 Tuas Avenue 2, Singapore) by an anesthesia consultant (AA) who was unaware of the group allocation. The cannula was secured in place with a transparent adhesive dressing (Tegaderm film, 3M Health Care, Neuss, Germany). The anesthesia registrar (AR) recorded the pain score as answered by the patient on a VAS scale of 0–10. The venipuncture site was assessed for the presence of skin erythema, swelling, and rashes at 12 h, 24 h, and at the time of decannulation.
All measured values were presented as mean ± standard deviation and numbers (%). The results were analyzed statistically using an unpaired Student's t-test and chi-square test/Fisher's exact test. Demographic data were analyzed with one way ANOVA for continuous variables and a chi square test for categorical variables. The incidence of venipuncture pain was analyzed with the chi square test; the VAS pain scores were analyzed with a Mann Whitney U test. The package SPSS 21 (SPSS Inc, Chicago, IL) was used for statistical analysis. A value of P < 0.05 was considered significant.
Go to :

RESULTS
The severity of pain as assessed by the VAS scores; the median (inter quartile range) was 6 (2) and 2 (2) for the control and ketoprofen group respectively (
P < 0.05) (
Table 2). Venipuncture pain was moderate in the control group as compared to the patients of the ketoprofen group, who had mild pain.
Table 2
Incidence and Severity of Venipuncture Pain


There was no incidence of erythema and swelling at the site of venous cannulation in either the ketoprofen group or the control group. Only one patient in Ketoprofen group developed a rash at the site of cannulation.
Go to :

DISCUSSION
This study has shown that pre-cannulation application of a ketoprofen patch decreases severity of venipuncture pain. The statistical interpretation of the data suggests that the application of a transdermal ketoprofen patch containing 20 mg of ketoprofen on the dorsum of the non-dominant hand 1 h before intravenous cannulation provides significant analgesia against venipuncture pain. Nearly all patients in the ketoprofen group felt pain as compared to the placebo group.
Venipuncture represents the beginning of the whole process of patient care by health care personnel. If this foundation is smooth and pain free, a lot of patient anxiety and perioperative stress can be allayed. Although the ideal target is to achieve “no pain” in 100% of patients on venipuncture, a ketoprofen patch is very helpful for the reduction of the severity of this pain.
Many pre-cannulation methods are being used to reduce venipuncture pain, like local anesthetic infiltration in skin, nitrous oxide inhalation, local application of EMLA, and non-steroidal anti-inflammatory agents [
345678]. Every method has its own advantages and disadvantages, for example EMLA is associated with local reactions like blanching, redness, contact dermatitis, hyper-pigmentation, and methemoglobinemia [
4].
The advantage of transdermal NSAIDs over oral preparation is that it attains a high concentration at the local site to produce the desired therapeutic effects without significant systemic absorption [
11]. Amongst NSAIDs, ketoprofen has several advantages as a transdermal preparation owing to its low molecular weight, low melting point, and high lipophilic nature. A low molecular weight is associated with elevated transdermal absorption. Amongst commonly used NSAIDs, the molecular weight of ketoprofen is 260 Dalton in comparison to 325 Dalton for diclofenac, 330 Dalton for piroxicam, and 350 Dalton for indomethacin [
13].
Ketoprofen, moreover, is one of the NSAIDs with the highest cutaneous permeability. Therefore, it penetrates the skin more rapidly when compared to other NSAIDs like diclofenac or indomethacin [
13]. In animal models, the ketoprofen patch preparation showed a superior skin permeability compared to diclofenac, flurbiprofen and piroxicam [
13]. Ketoprofen plaster has been used as a local application for the symptomatic relief of musculoskeletal pain like tendinitis, myalgia, scapula pain, and tennis elbow [
13].
Our study has the limitation that we did not use local anesthetic agent in the control group. We did not compare the analgesic efficacy of the ketoprofen patch to other proven treatment for venipuncture pain.
In conclusion, local application of the ketoprofen transdermal patch at the proposed site of intravenous cannulation one hour before the attempt is an effective, safe, and valid option for attenuating venipuncture pain in adults. Fewer incidences of edema and erythema at the venipuncture site is another advantage of this approach.
Go to :

ACKNOWLEDGEMENTS
Department of Anesthesiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Go to :

References
1. Keane TK, Hennis PJ, Bink-Boelkens MT. Non-drug related asystole associated with anaesthetic induction. Anaesthesia. 1991; 46:38–39. PMID:
1996753.

2. Hart PS, Yanny W. Needle phobia and malignant vasovagal syndrome. Anaesthesia. 1998; 53:1002–1004. PMID:
9893544.

3. Lal MK, McClelland J, Phillips J, Taub NA, Beattie RM. Comparison of EMLA cream versus placebo in children receiving distraction therapy for venepuncture. Acta Paediatr. 2001; 90:154–159. PMID:
11236044.

4. Agarwal A, Gautam S, Gupta D, Singh U. Transdermal diclofenac patch vs eutectic mixture of local anesthetics for venous cannulation pain. Can J Anaesth. 2007; 54:196–200. PMID:
17331931.

5. Smith AJ, Eggers KA, Stacey MR, Power I. Topical ibuprofen for skin analgesia prior to venepuncture. Anaesthesia. 1996; 51:495–497. PMID:
8694169.

6. Agarwal A, Yadav G, Gupta D, Tandon M, Dhiraaj S, Singh PK. Comparative evaluation of myolaxin and EMLA cream for attenuation of venous cannulation pain: a prospective, randomised, double blind study. Anaesth Intensive Care. 2007; 35:726–729. PMID:
17933159.

7. Usichenko TI, Pavlovic D, Foellner S, Wendt M. Reducing venipuncture pain by a cough trick: a randomized crossover volunteer study. Anesth Analg. 2004; 98:343–345. PMID:
14742367.

8. Agarwal A, Sinha PK, Tandon M, Dhiraaj S, Singh U. Evaluating the efficacy of the valsalva maneuver on venous cannulation pain: a prospective, randomized study. Anesth Analg. 2005; 101:1230–1232. PMID:
16192551.

9. Park HJ, Moon DE. Pharmacologic management of chronic pain. Korean J Pain. 2010; 23:99–108. PMID:
20556211.

10. Tripathi KD. Essentials of medical pharmacology. 6th ed. New Delhi: Jaypee Brothers;2008. p. 192–193.
11. Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev. 2010; CD007402. PMID:
20556778.

12. Komatsu T, Sakurada T. Comparison of the efficacy and skin permeability of topical NSAID preparations used in Europe. Eur J Pharm Sci. 2012; 47:890–895. PMID:
22985876.

13. Adachi H, Ioppolo F, Paoloni M, Santilli V. Physical characteristics, pharmacological properties and clinical efficacy of the ketoprofen patch: a new patch formulation. Eur Rev Med Pharmacol Sci. 2011; 15:823–830. PMID:
21780552.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download