Abstract
Background
Nefopam is a non-opioid, non-steroidal analgesic drug with fewer adverse effects than narcotic analgesics and nonsteroidal anti-inflammatory drugs, and is widely used for postoperative pain control. Because nefopam sometimes causes side effects such as nausea, vomiting, somnolence, hyperhidrosis and injection-related pain, manufacturers are advised to infuse it slowly, over a duration of 15 minutes. Nevertheless, pain at the injection site is very common. Therefore, we investigated the effect of warmed carrier fluid on nefopam injection-induced pain.
Methods
A total of 48 patients were randomly selected and allocated to either a control or a warming group. Warming was performed by diluting 40 mg of nefopam in 100 ml of normal saline heated to 31–32℃ using two fluid warmers. The control group was administered 40 mg of nefopam dissolved in 100 ml of normal saline stored at room temperature (21–22℃) through the fluid warmers, but the fluid warmers were not activated.
Results
The pain intensity was lower in the warming group than in the control group (P < 0.001). The pain severity and tolerance measurements also showed statistically significant differences between groups (P < 0.001). In the analysis of vital signs before and after the injection, the mean blood pressure after the injection differed significantly between the groups (P = 0.005), but the heart rate did not. The incidence of hypertension also showed a significant difference between groups (P = 0.017).
Nefopam is a non-opioid, non-steroidal analgesic drug with fewer adverse side effects as compared to those of narcotic analgesics and NSAIDs, and is widely used for postoperative pain control [1].
Because nefopam sometimes causes side effects such as nausea, vomiting, somnolence, hyperhidrosis, headache, blurred vision, and injection pain, manufacturers are advised to infuse it slowly, over 15 minutes. Nevertheless, pain at the injection site is very common. Furthermore, the slow infusion of nefopam can hinder the rapid resolution of acute pain.
In an attempt to address these problems, we investigated the effect of warmed carrier fluid on nefopam injection pain.
This prospective, randomized, single-blind, placebo-controlled study was approved by the Institutional Review Board of our hospital, and was performed after obtaining consent from all participating patients. The study included 20− to 70-year-old patients with American Society of Anesthesiologists physical status I and II, who underwent elective surgery under general anesthesia between January 2017 and February 2017. Only the cases where the catheter was more than 3 cm away from the elbow crease and proximal crease of the wrist in the anterior aspect of the forearm were included in the study (Fig. 1).
The exclusion criteria were as follows: American Society of Anesthesiologists class III or above; administration of other analgesic or vasoactive drugs; a history of cardiovascular, liver or kidney disease; basal heart rate > 100 beats/min; pain, redness or warm sensation in the intravenous catheterization site. In addition, cases in which the catheter was not cannulated in the anterior aspect of the forearm, was not an 18-gauge catheter, or was within 3 cm of the elbow crease or the proximal crease of the wrist were also excluded.
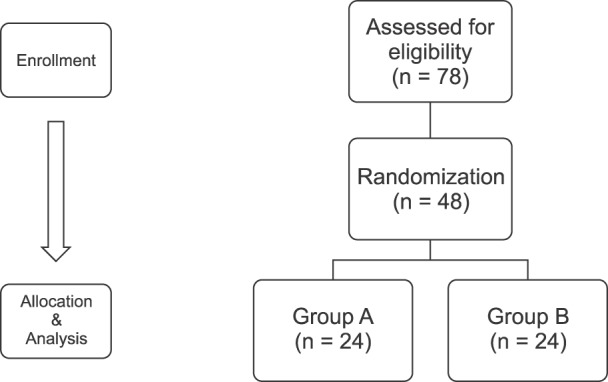
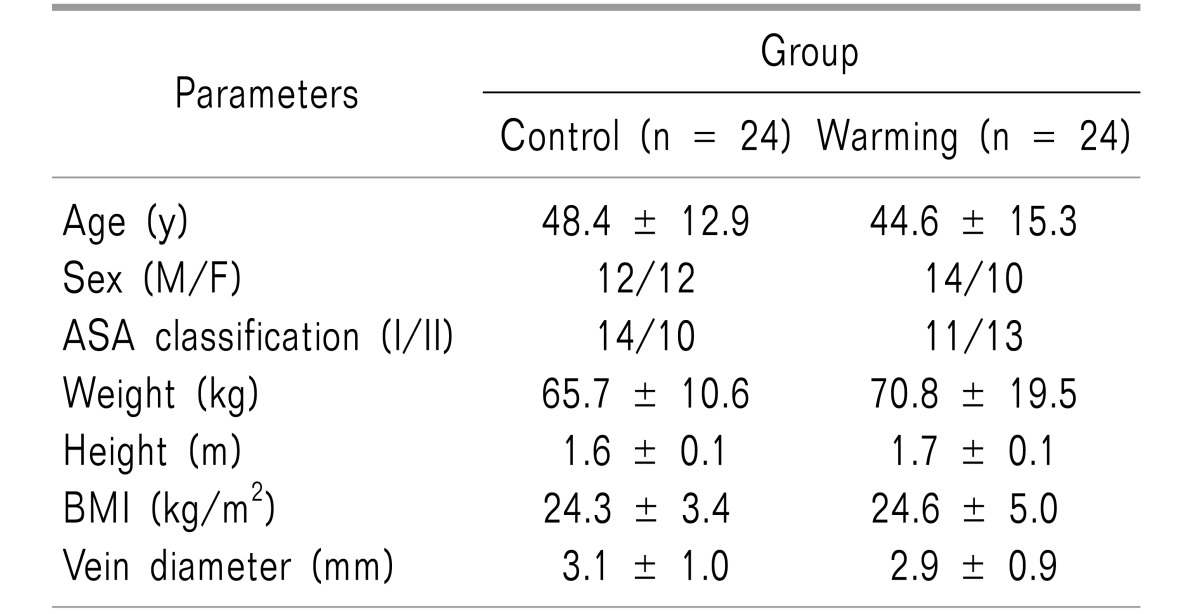
A total of 78 patients participated in this study (Fig. 2). Using a computer-generated random number table, 48 patients were selected for testing, of which 24 were assigned to each group, also using a computer-generated random number table (www.randomization.com). There were no statistically significant differences in demographic characteristics between the control and warming groups (Table 1).
The control group was administered nefopam 40 mg dissolved in 100 ml of normal saline stored at room temperature (21–22℃) through the fluid warmers, but the fluid warming was not activated. In the warming group, the same procedure was carried out, but with the fluid warmer devices activated. Warming was performed at 32–33℃ with two fluid warmers (Ranger™ Model 245 Blood/Fluid Warming Unit, Augustine Medical Inc., Eden Prairie, Minnesota, USA, and ANIMEC AM-2S-5A, ELLTEC, Nagoya, Aichi-ken, Japan) (Fig. 3). The power indicators of the fluid warmers were covered with black tape to prevent the patient or experimenter from knowing their operational status, so that the patients would not know which group they belonged to. In the warming group, all devices were preheated for 10 minutes before use to achieve optimum performance. In both groups, the infusion set was equally primed with experimental fluid, which was administered dropwise at a rate of 270 ml/h. Each experimental drug was administered as a premedication in the post-anesthetic care unit prior to transfer to the operating room.
Before starting nefopam infusion, mean blood pressure (MBP) and heart rate (HR) were checked; the drug was then administered. Each patient was asked to use a numerical rating scale (NRS; 0 = no pain, 10 = worst pain imaginable) to rate the maximum pain experienced during the 5-minute period following initiation of the injection. Pain severity was categorized and recorded according to the NRS score (0 = none, 1–3 = mild, 4–6 = moderate, 7–10 = severe). If the patient complained of pain at the injection site or asked to stop infusion, this was recorded as unendurable pain.
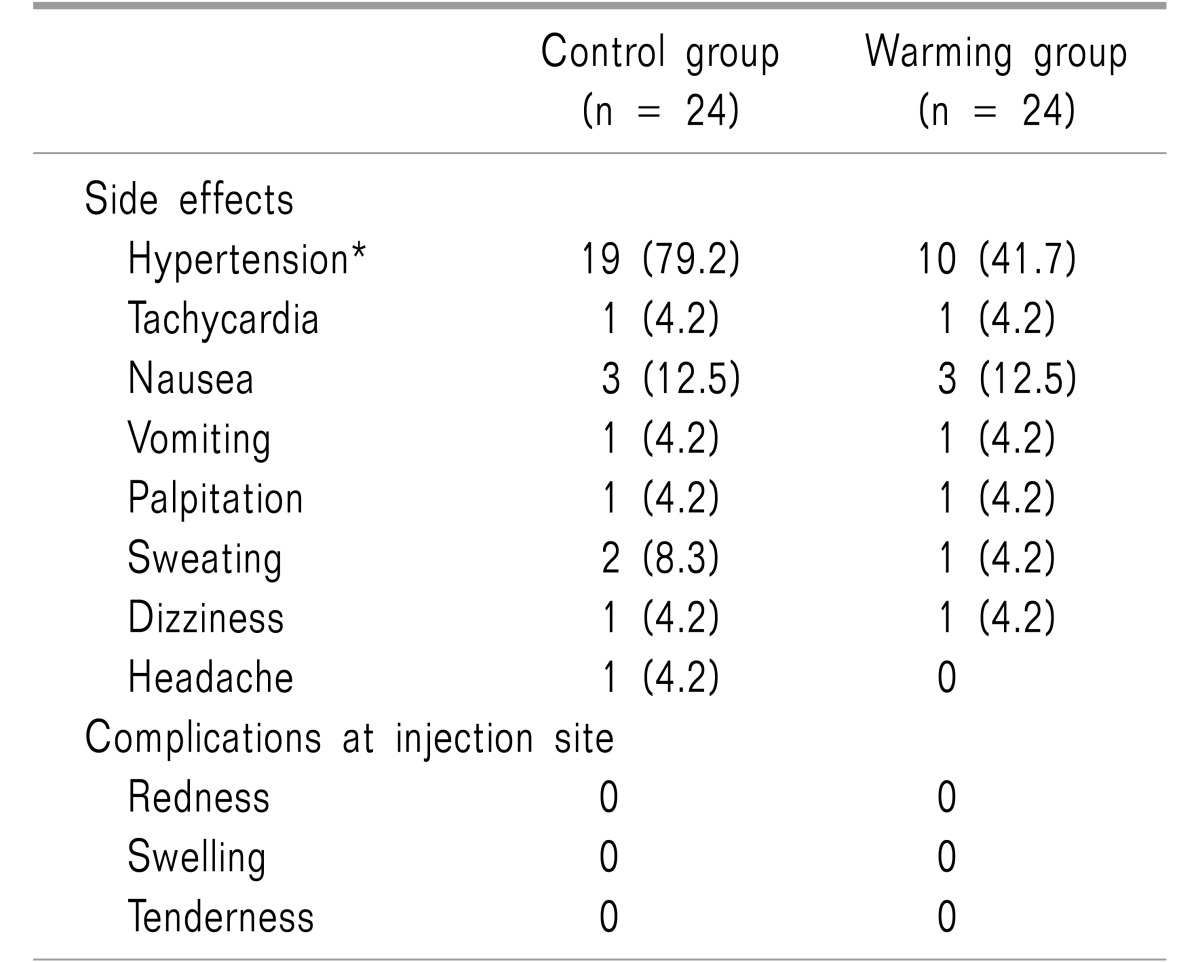
We noted any occurrence of redness, swelling, and tenderness at the intravenous injection site. Vein diameter was measured using ultrasonography by an anesthesiologist who was unaware of the study (Fig. 4). Also, side effects such as hypertension (systolic blood pressure > 140 mmHg or diastolic blood pressure < 50 mmHg), tachycardia (HR > 100 beats/min), palpitation, nausea, vomiting, sweating, dizziness, and headache were noted. If the patient complained of severe pain during the 5 min injection, the injection rate was halved so that the pain disappeared. Pain scores, side effects, and complications were recorded by an anesthesiologist who was not informed about the purpose of the study.
The primary endpoint was the post-injection NRS score, and the secondary endpoints were the pain severity, tolerance, and side effects. In a pilot study conducted to assist in setting the sample size, the average post-injection NRS scores of the control and warming groups were 3.97 ± 1.93 and 2.10 ± 1.28, respectively. Assuming α = 0.05 and power = 0.95, 21 patients were expected to be needed per group. The dropout rate was anticipated to be 10%, therefore the study was planned with a total of 48 patients, 24 from each group. Statistical analysis was done using SPSS v16.0 (SPSS Inc., Chicago, Illinois). The t-test or the Mann-Whitney U test were used for analyses of the NRS scores and changes in vital signs. A likelihood ratio test for trend was used for analysis of pain severity, and the Chi-square test or Fisher's exact test (if cell size ≤ 5) were used for analysis of pain tolerance and side effects. P values less than 0.05 were considered statistically significant.
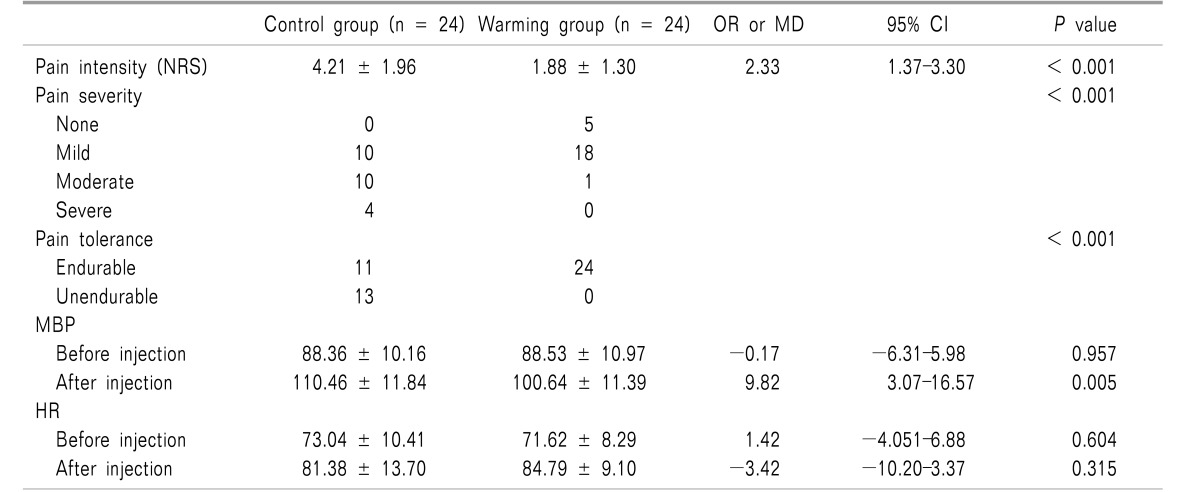
The pain intensity was lower in the warming group than in the control group (1.88 ± 1.30 vs. 4.21 ± 1.96, P < 0.001). The pain severity and tolerance measurements also showed statistically significant differences (P < 0.001).
In the analysis of vital signs before and after the injection, the MBP after injection was significantly different between the groups (P = 0.005), but the HR was not (Table 2).
The incidence of hypertension also showed a significant difference between groups (P = 0.017, Table 3).
This study showed that mildly warmed carrier fluid (31–32℃) significantly reduced pain during injection of nefopam. Compared to Kim et al.'s study [2], the use of warmed carrier fluid in this study allowed the same nefopam dose to be administered over less time (for 30 mg, 15 min vs. 20 min), and resulted in a faster infusion rate of nefopam, leading to minimal pain.
Nefopam has been widely used for the treatment and prevention of postoperative pain, because it has no effect on platelet function and no respiratory depression effect [345]. The mechanism of action of nefopam has not been fully explained; however, its central analgesic effect might be mediated by inhibiting the reuptake of serotonin, dopamine, and norepinephrine [67]. Since nefopam has several side effects, manufacturers are advised to infuse it slowly over 15 minutes to prevent side effects [8]. However, even this slow infusion rate causes side effects such as injection site pain, and furthermore makes it difficult to control acute pain, making clinicians reluctant to use the drug.
There have been many studies aimed at reducing the pain caused by injection of drugs such as propofol and rocuronium [91011]. Nefopam also causes injection pain, but research on it has not been sufficient. To date, only one study has been conducted to find the most rapid infusion rate of nefopam that does not cause injection pain and severe side effects. Kim et al. [2] compared infusion rates of 60 ml/h, 120 ml/h and 180 ml/h, and reported that the injection pain was lowest at 60 ml/h. They used 30 mg of nefopam diluted in 20 ml of normal saline (1.5 mg/ml), but the infusion rate was 60 ml/h, which required 20 minutes for complete infusion. The calculated rate (90 mg/h) was insufficient to control acute pain and was lower than that used in this study (108 mg/h). We referred to previous studies of the relationship between temperature and pain during injection [121314], in hopes of finding a way to infuse quickly with minimal pain.
All previous studies had used a temperature of about 40℃, so we also designed the study to use a warming fluid of 40℃. First, however, we needed to consider two factors related to patient safety: skin burns, and deterioration of the drug, caused by factors such as formation of precipitates or reduction of efficacy. Fortunately, previous studies have shown that a temperature of 40–43℃ does not cause skin burns or hyperthermia. In order to confirm the drug stability, we conducted the experiments and the experiments confirmed that the potency was not affected, and that denaturing did not occur at a temperature of 41℃.
In the pilot study, we observed that the temperature of the fluid, injected at a rate of 270 ml/h in a 21℃ operating room environment, dropped sharply as it passed through a relatively short 70 cm IV line (from 41℃ to 28℃). We found that carrier fluid at a temperature < 28℃ did not significantly reduce nefopam injection-induced pain (data not shown). The limitations of this Ranger™ fluid warmer have already been demonstrated in a previous study [15]. To solve this problem, an additional fluid warmer (ANIMEC) was installed in the IV line closer to the catheterized vein. We also needed to consider which type of external measurement would best reflect the actual temperature of the infused fluid. After several attempts, we found that the temperature of the IV line measured with an infrared thermometer 1.5 cm distal to the catheter best reflected the temperature at which it was actually injected (Fig. 5). Although no data were formally collected, the difference between the actual temperature of the infused fluid and the measured external temperature was observed to be < 1℃.
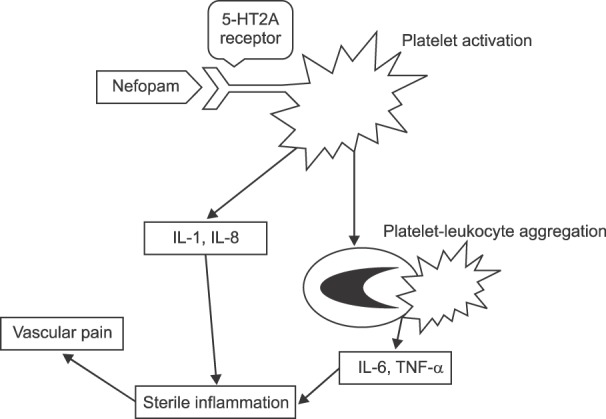
For propofol and rocuronium, it is known that injection pain is caused by inflammatory mediators such as histamine and kinin, or by activation of C-nociceptive fibers due to non-physiological osmotic pressure or pH [1216]. However, nefopam is almost isotonic, and has a pH of more than 5, which makes it difficult to attribute the pain it causes to non-physiological osmotic pressure or pH [2]. This suggests that consideration should be given to the receptor affinities of nefopam. A recent study showed that nefopam has a strong affinity for the 5-HT2A receptor [17]. Nefopam binds to the 5-HT2A receptors of platelets, and activates them without causing aggregation [318]. Activated platelets induce secretion of interleukin-1 (IL-1) and IL-8 in vascular endothelial cells, and at the same time bind to leukocytes that secrete IL-6 and tumor necrosis factor-alpha [1920]. Because these two pathways promote localized inflammation, it can be inferred that this is a mechanism by which nefopam induces vascular pain (Fig. 6). In our knowledge, this hypothesis is the first attempt to explain the mechanism of nefopam-induced pain.
Previous studies have suggested that injection pain is reduced by thermal vasodilation [121314]. While several studies reported vasodilatory effects after heating for more than 20 minutes [212223], it is doubtful whether heating for less than 5 minutes caused vasodilatory effects in previous studies, or in this study. On the other hand, we know that mildly cool fluid (< 22℃) induces sustained vasoconstriction, while a thermoneutral temperature (24–32℃) is expected to alleviate vasoconstriction [2425]. It is presumed that the relative coolness of the fluid enhanced vasoconstriction, allowing activated platelets and platelet-leukocyte complexes to make better contact with the endothelium, thereby resulting in more severe pain than that in the warming group.
In a quantitative systematic review, the use of nefopam increased the risks of tachycardia and sweating: the incidences were 21.3% and 8.8%, respectively [5]. In this study, the incidence of tachycardia was about 4%, suggesting that either the concentration or infusion rate of nefopam were different. Patients in both groups from this study showed a high incidence of hypertension (> 40%), probably due to a slight inotropic effect of nefopam [26]. MBP was significantly higher in the control group after nefopam injection than in the warming group; it is presumed that the sympathetic nervous system was further stimulated by the greater pain intensity [27]. Therefore, the use of nefopam in patients requiring precise blood pressure control deserves caution.
Previous studies have suggested ways to reduce injection pain, but they had limitations. First, the diameter of vessels and the catheter insertion site were not controlled. These two factors need to be controlled because they affect the pain experienced during injection. Second, the actual injection temperature was not measured, or the measurement site was not specified in detail. Because we eliminated variability by controlling these factors, we can assert that this study had significantly advantages over previous studies. However, our research design also had drawbacks: it did not evaluate the changes in vessel diameter caused by heating or cooling. Future research will be needed to directly measure and verify the vasomotor effects produced by temperature. In addition, it is difficult to use several heating devices simultaneously in clinical practice. Since the beneficial effect of warmed carrier fluid has been demonstrated in this study, development of an efficient IV-line heating device would help reduce injection-related pain.
In conclusion, use of warmed carrier fluid for nefopam injection decreased injection-induced pain compared to mildly cool carrier fluid.
References
1. Girard P, Chauvin M, Verleye M. Nefopam analgesia and its role in multimodal analgesia: a review of preclinical and clinical studies. Clin Exp Pharmacol Physiol. 2016; 43:3–12. PMID: 26475417.

2. Kim YM, Lim BG, Kim H, Kong MH, Lee MK, Lee IO. Slow injection of nefopam reduces pain intensity associated with intravenous injection: a prospective randomized trial. J Anesth. 2014; 28:399–406. PMID: 24201414.

3. Dordoni PL, Della Ventura M, Stefanelli A, Iannace E, Paparella P, Rocca B, et al. Effect of ketorolac, ketoprofen and nefopam on platelet function. Anaesthesia. 1994; 49:1046–1049. PMID: 7864317.

4. Bhatt AM, Pleuvry BJ, Maddison SE. Respiratory and metabolic effects of oral nefopam in human volunteers. Br J Clin Pharmacol. 1981; 11:209–211. PMID: 6783057.

5. Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008; 101:610–617. PMID: 18796441.

6. Piercey MF, Schroeder LA. Spinal and supraspinal sites for morphine and nefopam analgesia in the mouse. Eur J Pharmacol. 1981; 74:135–140. PMID: 6276187.

7. Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS. Nefopam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980; 19:249–267. PMID: 6991238.
8. Durrieu G, Olivier P, Bagheri H, Montastruc JL. Overview of adverse reactions to nefopam: an analysis of the French Pharmacovigilance database. Fundam Clin Pharmacol. 2007; 21:555–558. PMID: 17868209.

9. Jung KT, Kim HJ, Bae HS, Lee HY, Kim SH, So KY, et al. Effects of lidocaine, ketamine, and remifentanil on withdrawal response of rocuronium. Korean J Anesthesiol. 2014; 67:175–180. PMID: 25302093.

10. Polat R, Aktay M, Ozlü O. The effects of remifentanil, lidocaine, metoclopramide, or ketamine pretreatment on propofol injection pain. Middle East J Anaesthesiol. 2012; 21:673–677. PMID: 23265029.
11. Alipour M, Tabari M, Alipour M. Paracetamol, ondansetron, granisetron, magnesium sulfate and lidocaine and reduced propofol injection pain. Iran Red Crescent Med J. 2014; 16:e16086. PMID: 24829787.

12. Mahajan C, Rath GP, Bithal PK, Prabhakar H, Yadav R, Dube SK. Local warming at injection site helps alleviate pain after rocuronium administration. J Anesth. 2010; 24:845–848. PMID: 20737278.

13. Jeong M, Yoon H. Comparison of the effects of lidocaine pre-administration and local warming of the intravenous access site on propofol injection pain: randomized, double-blind controlled trial. Int J Nurs Stud. 2016; 61:209–218. PMID: 27372434.

14. Youn AM, Hsu TM. Heated carrier fluids in decreasing propofol injection pain: a randomized, controlled trial. Korean J Anesthesiol. 2017; 70:33–38. PMID: 28184264.

15. Kim DJ, Kim SH, So KY, An TH. Mega Acer Kit® is more effective for warming the intravenous fluid than Ranger™ and ThermoSens® at 440 ml/h of infusion rate: an experimental performance study. Korean J Anesthesiol. 2017; 70:456–461. PMID: 28794842.

16. Klement W, Arndt JO. Pain on i.v. injection of some anaesthetic agents is evoked by the unphysiological osmolality or pH of their formulations. Br J Anaesth. 1991; 66:189–195. PMID: 1817619.

17. Gregori-Puigjane E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, et al. Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci U S A. 2012; 109:11178–11183. PMID: 22711801.

18. Raote I, Bhattacharya A, Panicker MM. Serotonin 2A(5-HT2A) receptor function: ligand-dependent mechanisms and pathways. In : Chattopadhyay A, editor. Serotonin receptors in neurobiology. Boca Raton (FL): CRC Press;2007. p. 105–132.
19. Kaplanski G, Porat R, Aiura K, Erban JK, Gelfand JA, Dinarello CA. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood. 1993; 81:2492–2495. PMID: 8490165.

20. Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, et al. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation. 1997; 95:2387–2394. PMID: 9170401.

21. Christen S, Delachaux A, Dischl B, Golay S, Liaudet L, Feihl F, et al. Dose-dependent vasodilatory effects of acetylcholine and local warming on skin microcirculation. J Cardiovasc Pharmacol. 2004; 44:659–664. PMID: 15550784.

22. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985). 2001; 91:1619–1626. PMID: 11568143.

23. Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol Respir Environ Exerc Physiol. 1984; 57:191–196. PMID: 6469780.

24. Elstad M, Vanggaard L, Lossius AH, Walløe L, Bergersen TK. Responses in acral and non-acral skin vasomotion and temperature during lowering of ambient temperature. J Therm Biol. 2014; 45:168–174. PMID: 25436967.

25. Bergersen TK, Skytioti M, Elstad M. Cold-induced sympathetic tone modifies the impact of endothelium-dependent vasodilation in the finger pulp. Auton Neurosci. 2017; 203:97–102. PMID: 27932205.

26. Hagemann K, Platte G, Meyer J, Effert S. Haemodynamic effects of nefopam (author's transl). Dtsch Med Wochenschr. 1978; 103:1040–1043. PMID: 668511.
27. Sacco M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, et al. The relationship between blood pressure and pain. J Clin Hypertens (Greenwich). 2013; 15:600–605. PMID: 23889724.

Fig. 3
Fluid warming devices used in this study. Fluid warmer device 1: Ranger™ Model 245 Blood/Fluid Warming Unit, Augustine Medical Inc., USA. Fluid warmer device 2: ANIMEC AM-2S-5A, ELLTEC, Japan.
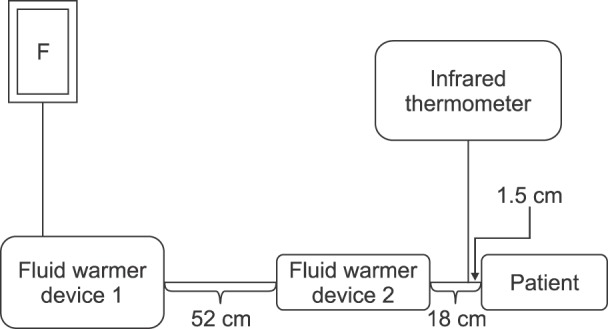
Fig. 5
Illustration of a fluid warmer and measurement of infusion fluid temperature using an infrared thermometer. This picture is from a pilot study. In the study itself, the power indicator of the ANIMEC warmer was covered with black tape.
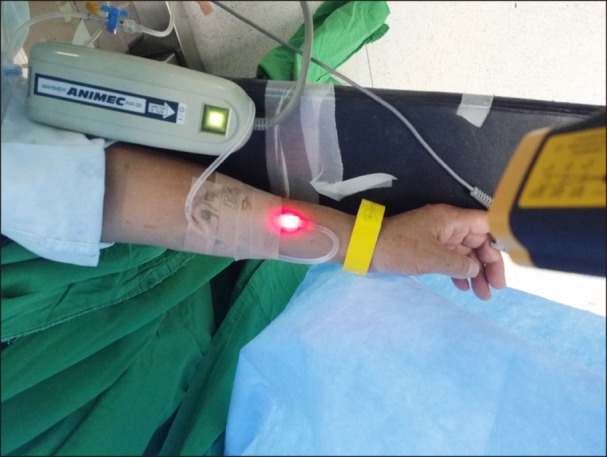
Fig. 6
Diagram illustrating hypothesis concerning linkage between platelet activation and vascular pain.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download