INTRODUCTION
MAIN BODY
1. History for approved indications
2. Mechanism of action matching with bone remodeling cycle
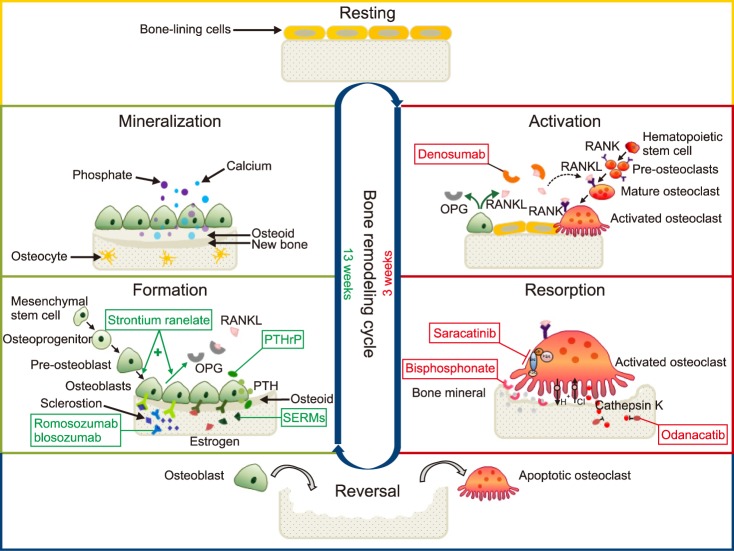 | Fig. 1Bone remodeling cycle and medications for the treatment of osteoporosis. Bone remodeling cycle consists of at least 6 different phases: 1. resting, 2. activation, 3. resorption, 4. reversal, 5. formation, and 6. mineralization. Drugs for the treatment or prevention of osteoporosis act on different phases. In the activation phase, denosumab, like OPG (a decoy receptor produced by osteoblasts), binds the RANKL and blocks the RANKL from binding to the RANK, inhibits the differentiation steps from pre-osteoblasts via mature osteoclasts to activated osteoclasts, and finally reduces bone resorption. In the resorption phase, bisphosphonates bind to the bone mineral and take the space where activated osteoclasts attach at sites of bone resorption. Odanacatib, a cathepsin K inhibitor, inhibits the osteoclastic enzyme that degrades collagens. Saracatinib, a c-src inhibitor, inhibits osteoclastic activation. Selective estrogen receptor modulators (SERMs) and hormone (estrogen) replacement therapy interfere with various osteoblast-derived factors that stimulate osteoclasts. In the formation phase, strontium ranelate stimulates pre-osteoblasts to differentiate into osteoblasts, and stimulates osteoblasts to secrete OPG in order to prevent pre-osteoclasts from becoming activated osteoclasts via mature osteoclasts, as well. Parathyroid hormone (PTH) analogues and PTH-related protein (PTHrP) analogues increase the number and activity of osteoblasts. Romosozumab (AMG 785) and blosozumab, anti-sclerostin monoclonal antibodies, bind to the sclerostin (a glycoprotein inhibitor of osteoblast Wnt signaling produced by osteocytes) and inhibit its action. Cbl: Casitas B-lineage lymphoma, FAK: focal adhesion kinase, OPG: osteoprotegerin, PI3k: phosphoinositide 3-kinase, PTH: parathyroid hormone, PTHrP: PTH-related protein, RANK: the nuclear factor kappa B, RANKL: RANK ligand, SERMs: selective estrogen receptor modulators, Src: Src family kinase (a group of non-receptor tyrosine kinases). Modified from Connelly D. Osteoporosis: moving beyond bisphosphonates. Pharmaceutical Journal 2016 Nov [2016 Nov 23]. Available at http://www.pharmaceutical-journal.com/news-and-analysis/infographics/osteoporosis-moving-beyond-bisphosphonates/20201978.article |
3. Pharmacodynamics
4. Pharmacokinetics
5. Precautions related to contraindications and complications
1) Contraindications
2) Complications
6. Preparation and administration methods
7. Future development in the use of denosumab
CONCLUSIONS
Table 2
Differences between Denosumab and Bisphosphonates

BMD: bone mineral density, CTX: C-terminal telopeptide, FPP: farnesyl pyrophosphate, OPG: osteoprotegerin, RANK: receptor activator of the nuclear factor kappa B, RANKL: receptor activator of the nuclear factor kappa B ligand. Modified from Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48: 677-92.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download