Abstract
Background
The root of Peucedanum japonicum Thunb., a perennial herb found in Japan, the Philippines, China, and Korea, is used as an analgesic. In a previous study, sec-O-glucosylhamaudol (SOG) showed an analgesic effect. This study was performed to examine the antinociceptive effect of intrathecal SOG in the formalin test.
Methods
Male Sprague-Dawley rats were implanted with an intrathecal catheter. Rats were randomly treated with a vehicle and SOG (10 µg, 30 µg, 60 µg, and 100 µg) before formalin injection. Five percent formalin was injected into the hind-paw, and a biphasic reaction followed, consisting of flinching and licking behaviors (phase 1, 0–10 min; phase 2, 10–60 min). Naloxone was injected 10 min before administration of SOG 100 µg to evaluate the involvement of SOG with an opioid receptor. Dose-responsiveness and ED50 values were calculated.
Results
Intrathecal SOG showed a significant reduction of the flinching responses at both phases in a dose-dependent manner. Significant effects were showed from the dose of 30 µg and maximum effects were achieved at a dose of 100 µg in both phases. The ED50 value (95% confidence intervals) of intrathecal SOG was 30.3 (25.8–35.5) µg during phase 1, and 48.0 (41.4–55.7) during phase 2. The antinociceptive effects of SOG (100 µg) were significantly reverted at both phases of the formalin test by naloxone.
From over 7000 years ago, various natural products have been utilized to treat pain disorders. Substances derived from natural products, such as opioids, cannabinoids, and vanilloids, have been utilized to treat pain disorders [1]. Still, in the present day, the pharmaceutical industry is interested in the screening of natural products, and academic research is still focused on discovering new chemical entities from natural products [1].
The root of Peucedanum japonicum Thunb., also known as coastal hogfennel, is a species in the genus Peucedanum (family of Umbelliferae) which is found in Japan, the Philippines, China, and Korea [2]. In Korea, it is usually referred to as ‘bangpung’ and used as a main ingredient in salads. It is a perennial herb which was used for cough, colds, headaches, and neuralgic disease [23]. In a previous study, it was discovered that the Peucedanum species have bioactivities such as antioxidant activity [4], antiplatelet aggregation [3], anti-inflammatory activity [5] and anti-inflammatory activity on COX-1 and COX-2 [6].
The constituents isolated from Peucedanum japonicum Thunb. are known as Psoralen, Scopoletin, Cimifugin, lsoimperatorin, (+)-Marmesin, Xanthotoxin, Hamaudol, Daucosterol, Galactitol, Sec-O-glucosylhamaudol (SOG), and etc. [6]. In the previous study, SOG which was isolated from the root of Saposhnikovia divaricate (the family of Umbelliferae), showed an analgesic activity [7]. Thus, we were interested in the traditional use of Peucedanum japonicum Thunb. for headaches and neuralgia, and in the analgesic activity of SOG which is isolated from Peucedanum japonicum Thunb.. This study was performed to examine the antinociceptive effect of intrathecal SOG in the formalin test and to evaluate the involvement of SOG on the opioid receptor.
This study was approved by the Institutional Animal Care and Use Committee of Chonnam National University. The protocol and the experiments followed the International Association for the Study of Pain guidelines on ethical standards for the investigation of experimental pain in animals [8]. Male Sprague-Dawley rats weighing 225-250 g were used for the experiments (n = 36). The animals were raised in a room with constant temperature (20 to 23℃) and a 12-hour light/dark cycle. Water and food were allowed with no limitations.
The rats were anesthetized for the implantation of a polyethylene-5 (PE-5) catheter into the intrathecal space. A PE-5 catheter was implanted via the cisterna magna for drug administration [9]. When the rats were anesthetized with sevoflurane, the atlanto-occipital membrane was incised after sterile dressing, and the catheter was advanced caudally by 8.5 cm to place the end of catheter on the level of lumbar enlargement. Then, the other end of the catheter was externalized through the skin of the head and anchored firmly by suture for drug administration in the future. The tip of the externalized catheter was plugged with a stainless steel wire and the rats were kept in individual cages until they awoke from the anesthesia, and then the signs of neurologic dysfunction were observed. The rats with a neurologic dysfunction such as limping after the catheter implantation were killed immediately with an overdose of inhalational anesthetics. The rats without neurologic deficits were sent back to the vivarium and housed in individual cages after surgery.
Sec-O-glucosylhamaudol (purity > 95%) was purchased from the Natural Product Bank (Gyeongsangbuk-do, Korea). SOG was dissolved in 60% dimethylsulfoxide (DMSO) and diluted as 10, 30, 60, and 100 µg.
The rats were restrained in a cylinder and held for 20 min for adaptation, and SOG or vehicle was administered intrathecally through the intrathecal catheter with a gear-operated Hamilton syringe pump 10 m prior to the formalin injection. SOG was delivered in a volume of 10 µl solution, followed by an additional 10 µl of saline to flush the catheter. Different doses of SOG were randomly given or 100% DMSO were given as a control.
The formalin test was conducted 5 days after the surgery to permit recovery from the intrathecal catheterization. The person who carried out the behavioral testing was blind to the treatment. The rats were restrained in a cylinder and held for 20 m for adaptation. Then, 50 µl of 5% formalin was injected with a 30 gauge needle into the center of the hind-paw of the rat subcutaneously. Flinching responses were evaluated by counting the number of responses during 1 m periods at 1 and 5 m after the formalin injection for the initial acute phase (phase 1, 0-9 m), and every 5 m up to 60 m thereafter (phase 2, 10-60 m) [10].
Dose-responsiveness and ED50 (a dose that produced a 50% reduction in the number of flinches compared with the vehicle) values were calculated. Dose-response data are calculated as the percentage of control in the two phases as follows;
ED50 of SOG and its confidence interval were calculated using a standard linear regression analysis of a dose-response curve, according to the method by Tallarida [11].
Next, rats were pretreated with naloxone in order to determine the involvement of SOG on the opioid receptors. Naloxone (0.3 µg) was administered intrathecally 10 m before SOG administration (100 µg) and the formalin was injected 10 m later SOG administration.
All data are expressed as mean ± SEM. The time-response data or the dose–response data are expressed either as the number of flinches or the percentage of control in two phases. Data from phase 1 and phase 2 were analyzed separately because the formalin test caused a distinct biphasic flinching response. Statistical analysis was done using one-way analysis of variance followed by a post hoc test with Turkey's test for multiple comparisons. Values with P < 0.05 were considered to be statistically significant.
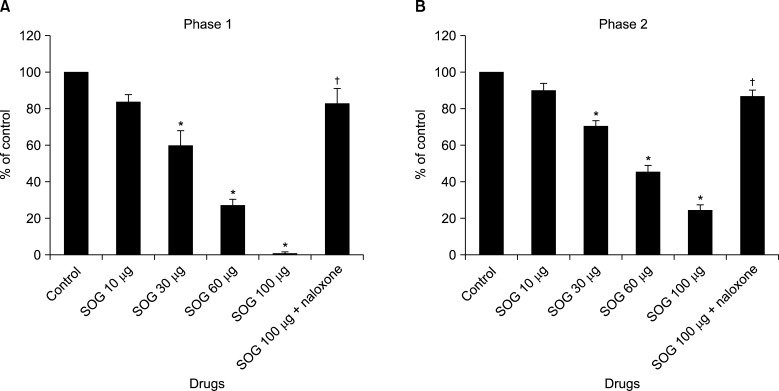
The rats showed the typical biphasic flinching responses after the formalin injection when treated with an intrathecal vehicle (Fig. 1). Intrathecal SOG administration 10 min prior formalin injection showed a significant reduction of the flinching responses at both phases in a dose-dependent manner (Fig. 1 and 2). The antinociceptive effect of intrathecal SOG was dose-dependent, but significant effects were showed from the dose of 30 µg in both phases (phase 1; 60.2% of control, phase 2; 70.6% of control, P < 0.001, Fig. 2). Maximum effects were achieved at a dose of 100 µg in both phases (phase 1; 1.2% of control, phase 2; 24.6% of control, P < 0.001, Fig. 2). The ED50 value (95% confidence intervals) of intrathecal SOG was 30.3 (25.8-35.5) µg during phase 1, and 48.0 (41.4-55.7) during phase 2.
In the current study, intrathecal SOG significantly reduced the flinching responses at both phases in a dose-dependent manner and the effect of SOG was significantly reverted at both phases of the formalin test by naloxone. These results suggest that intrathecal SOG has an antinociceptive effect which is related to the opioid receptor in the formalin test.
The species of Peucedanum are rich in coumarins and essential oils, and also contains some phenolic acids, flavonoids, terpenoids and other components [12]. A total of 158 coumarins, 13 flavonoids, 13 phenolic acids, 11 phenylpropanoids, 9 fatty acids, 8 chromones, 2 steroids, and a number of volatile have been identified from the Peucedanum species. The main constituents of the Peucedanum species are coumarins and essential oils, and they are considered to be responsible for a wide spectrum of the biological and pharmacological activities of the Peucedanum species [12]. According to the previous studies, the traditional analgesic effect of Peucedanum japonicum Thunb. could be related to its bioactivities such as antioxidant activity (3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, and cnidioside) [4], anti-inflammatory activity (p-chloro-benzylamide and N-methyl-piperazide) [5], and actions on COX-1 and COX-2 (psoralen, scopoletin, and hamaudol) [6]. Especially, p-chloro-benzylamide and N-methyl-piperazide suppressed the carrageenan-induced inflammation in mice [5].
However, there were no reports about SOG until 2001. SOG was first reported as a new chromone, a derivative of benzopyran with a substituted keto group on the pyran ring. It is an isomer of coumarin which was isolated from Ledebouriella seseloides (family of Umbelliferae) in 1982 [13]. Okuyama et al. [7] has reported that SOG has antiallodynic effects. Thus, we focused on SOG and performed experiments to evaluate the antinociceptive effect of intrathecal SOG in the formalin test and its relationship with the opioid receptor.
In the study of Okuyama et al. [7], they administered SOG 80 mg/kg orally and the pain threshold increased significantly in the tail pressure examination. SOG also increased the pain threshold of inflamed and non-inflamed hind paws in the modified Randall & Selitto test. However, SOG did not show a significant hypothermic effect on lipopolysaccharide-induced pyrexia at doses of 80 and 160 mg/kg. And, the analgesic effect of SOG was reversed by subcutaneous injection of naloxone. Thus, they suggested that SOG affects the central nervous system and the analgesic pathway of SOG is related to an opioid receptor.
These results are agreed with our data. In the current study, intrathecal SOG showed antinociceptive effect for both phases in a dose-dependent manner. It is known that the response to formalin shows an early and a late phase. The response in phase 1 results from direct stimulation of nociceptors and the response in phase 2 appears to be dependent on the combination of an inflammatory reaction in the peripheral tissue and functional changes in the dorsal horn of the spinal cord [14]. Thus, anti-inflammatory drugs suppress flinching responses only in phase 2 and opiod analgesics appear to be antinociceptive for both phases [14]. SOG suppressed flinching responses in both phases, therefore, it is suggested that the effect of SOG is related to an opioid receptor. Moreover, the maximal antinociceptive effect of SOG, when administered 100 µg intrathecally, was significantly reverted at both phases of the formalin test by naloxone which was administered 10 min before the delivery of SOG.
Notably, SOG showed very significant dose-dependent antinociceptive effects after the formalin injection. Maximum effects in both phases were presented at a dose of 100 µg (phase 1; 1.2% of control, phase 2; 24.6% of control, P < 0.001, Fig. 2). These effects are similar or greater than the effects of intrathecal morphine when compared with previous studies [1516]. From this fact it seems that SOG has a very strong antinociceptive effect which is related to an opioid receptor.
There were some limitations in this study. First, we only examined the effect of SOG by behavioral study. Further evaluations such as molecular work are needed to examine the exact mechanism of SOG and its role with the opioid receptor. Second, SOG may also have an anti-inflammatory effect like other constituents of the Peucedanum species. Further evaluations of its anti-inflammatory effect and its effects against inflammatory pain are needed. Third, SOG has a very strong antinociceptive effect which is related to an opioid receptor. However, we did not evaluate the associated side-effects of opioids receptors such as agitation, loss of the pinna reflex, motor disturbance, and flaccidity [16]. Further evaluations about side-effects of SOG are needed for clinical application. Fourth, DMSO itself can reduce flinching responses after a formalin injection in both phases [17]. SOG is a hydrophobic substances and could not dissolved in normal saline. Therefore, DMSO had to be used as a solvent. However, SOG which was dissolved in DMSO significantly decreased the flinching responses after the formalin injection more than when DMSO alone was used. This may be evidence that SOG has an antinociceptive effect. However, another limitation to consider is that DMSO can affect the effectiveness of SOG.
In conclusion, intrathecal SOG significantly decreased the flinching responses after the formalin injection at both phases in a dose-dependent manner, and the antinociceptive effects were reverted by naloxone. SOG has a very strong antinociceptive effect and it seems the effect is related to an opioid receptor.
References
1. McCurdy CR, Scully SS. Analgesic substances derived from natural products (natureceuticals). Life Sci. 2005; 78:476–484. PMID: 16216276.

2. Ikeshiro Y, Mase I, Tomita Y. Dihydropyranocoumarins from roots of Peucedanum japonicum. Phytochemistry. 1992; 31:4303–4306.

3. Chen IS, Chang CT, Sheen WS, Teng CM, Tsai IL, Duh CY, et al. Coumarins and antiplatelet aggregation constituents from Formosan Peucedanum japonicum. Phytochemistry. 1996; 41:525–530. PMID: 8821432.

4. Hisamoto M, Kikuzaki H, Ohigashi H, Nakatani N. Antioxidant compounds from the leaves of Peucedanum japonicum thunb. J Agric Food Chem. 2003; 51:5255–5261. PMID: 12926867.

5. Zimecki M, Artym J, Cisowski W, Mazol I, Włodarczyk M, Gleńsk M. Immunomodulatory and anti-inflammatory activity of selected osthole derivatives. Z Naturforsch C. 2009; 64:361–368. PMID: 19678539.

6. Zheng M, Jin W, Son KH, Chang HW, Kim HP, Bae KH, et al. The constituents isolated from Peucedanum japonicum Thunb. and their cyclooxygenase (COX) inhibitory activity. Korean J Med Crop Sci. 2005; 13:75–79.
7. Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi M, Yamazaki M. Analgesic components of saposhnikovia root (Saposhnikovia divaricata). Chem Pharm Bull (Tokyo). 2001; 49:154–160. PMID: 11217101.

8. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16:109–110. PMID: 6877845.

9. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036. PMID: 14677603.

10. Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996; 64:345–355. PMID: 8740613.

11. Tallarida RJ. Drug synergism and dose-effect data analysis. Boca Raton (FL): Chapman & Hall/CRC;2000. p. 57–71.
12. Sarkhail P. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: a review. J Ethnopharmacol. 2014; 156:235–270. PMID: 25193684.

13. Sasaki H, Taguchi H, Endo T, Yosioka I. The constituents of Ledebouriella seseloides Wolff. I. structures of three new chromones. Chem Pharm Bull (Tokyo). 1982; 30:3555–3562.

14. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987; 30:103–114. PMID: 3614974.

15. Yoon MH, Park KD, Lee HG, Kim WM, An TH, Kim YO, et al. Additive antinociception between intrathecal sildenafil and morphine in the rat formalin test. J Korean Med Sci. 2008; 23:1033–1038. PMID: 19119449.

16. Nishiyama T. Interaction between intrathecal morphine and glutamate receptor antagonists in formalin test. Eur J Pharmacol. 2000; 395:203–210. PMID: 10812050.

17. Colucci M, Maione F, Bonito MC, Piscopo A, Di Giannuario A, Pieretti S. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res. 2008; 57:419–425. PMID: 18508278.

Fig. 1
Time course after formalin injection shows antinociceptive effects of intrathecal sec-O-glucosylhamaudol (SOG). SOG reduced the flinching responses after formalin injection significantly in a dose dependent manner during both phases. The antinociceptive effect of SOG (100 µg) was significantly reverted at both phases of formalin test by naloxone. Each line represents the mean ± SEM of 6 rats/group.
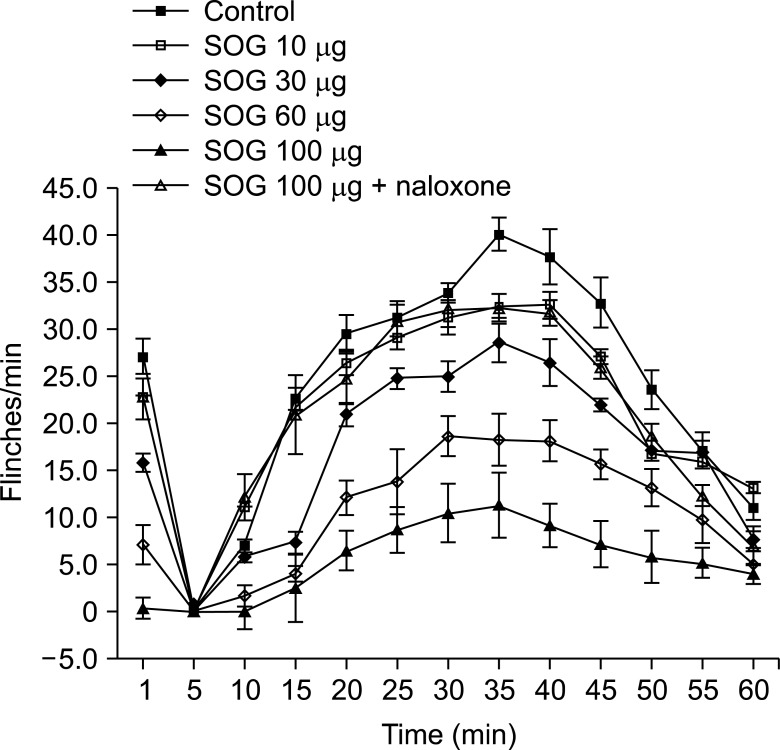
Fig. 2
Dose–response data shows antinociceptive effects of intrathecal sec-O-glucosylhamaudol (SOG). SOG reduced the flinching responses after formalin injection significantly in a dose dependent manner during phase 1 (A) and phase 2 (B). Significant effect was showed from the dose of 30 µg and maximum effect was achieved at a dose of 100 µg both phases. The antinociceptive effect of SOG (100 µg) was significantly reverted at both phases of formalin test by naloxone. Data are presented as the percentage of control. *P < 0.001 vs. control. †P < 0.001 vs. SOG 100 µg.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download