Abstract
The lumbar sympathetic ganglion block (LSGB) is widely used for diagnosing and treating sympathetically maintained pain disorders. The LSGB has been conventionally carried out under fluoroscopy or computed tomography guidance. However, as ultrasound technology improved, ultrasound-guided interventions have been expanding their territory to deeper structures. Ultrasound guidance provides many benefits including protecting vascular injection, shortening procedure time in some cases, and reducing the emission of radiation. In this report, we describe a successful case of a US-guided LSGB without major complications. We expect that US-guided LSGBs can be implemented and furnished in the daily outpatient clinical setting by highly trained pain physicians.
The lumbar sympathetic ganglion (or chain) block (LSGB) is widely used for diagnosing and treating sympathetically maintained pain [12]. Usually, LSGBs are carried out under fluoroscopy guidance, and more recently, with the assistance of computed tomography (CT) [3]. However, they require the use of an equipped operating room or the aid of a radiology department in cases of computed tomography, with radiation exposure at a higher level with repeated use [4].
In previous studies, US-guided interventions have shown many benefits including a reduction in radiation exposure and enabling viewing of dynamic images of soft tissues such as muscles, tendons, nerves, and vessels [567]. Furthermore, the real-time nature of the technique under US can confirm the spread of injectate. For these reasons, the stellate ganglion block is nowadays mostly performed under ultrasound guidance [7]. The celiac plexus block (CPB) and superior hypogastric plexus block have also been tried under ultrasound guidance successfully [89].
As far as we know, there is only one article regarding LSGBs guided by ultrasound, but the paper is out of date and the explanation and description of the technique in the paper was insufficient [10]. Therefore, in this brief report, we describe a case of a US-guided LSGB with technical details, which was performed successfully without complications.
A twenty year-old female (50 kg, 160 cm) was referred to our pain clinic due to left ankle pain, 2 years after a pedestrian traffic accident, and after prior failed attempts at pain control and functional rehabilitation through physical therapy and medical management. Prior to this injury the patient was an active 18-year-old girl without any medical history. The patient suffered throbbing, tearing-apart like, and stabbing pain around her left ankle with marked allodynia on her left sole with 8/10 of an 11-pointed numerical rating scale (NRS) pain score in severity. We evaluated the patient using a standardized assessment protocol for diagnosing CRPS as described by the Budapest research criteria [11], and diagnosed her with CRPS type I affecting her left foot. Apart from oral medication such as antidepressants, anticonvulsants, and tramadol, she has received an LSGB on the left side at every monthly follow-up. After the LSGBs, she has experienced a significant reduction in pain from 8 to 5 or 6; however, during fluoroscope-guided LSGBs, she could not tolerate procedure-related pain due to the needle tip touching her L3 vertebra's bony cortex and periosteum. In an attempt to avoid the pain during the LSGB, we planned to perform the LSGB under ultrasound guidance using fluoroscopic confirmation.
A portable ultrasound image device (HD11XE, Koninklijke Philips N.V. Amsterdam, the Netherlands) with 5–2 MHz low frequency round probe was used. The patient entered the operation room with a 24 G intravenous route, and non-invasive blood pressure and pulse oxygen saturation level were continuously monitored during and after the procedure. She was placed in a prone position with a pillow under the lower abdomen and iliac crest to reduce lumbar lordosis, and temperature probes were attached to both soles using transparent patches (Teraderm™, 3M Healthcare, St Paul, MN, USA). After sterilizing and draping the skin around the puncture sites, both soles were also covered to stabilize temperature. The LSGB was performed at the upper third of the L3 vertebra without any pre-medication.
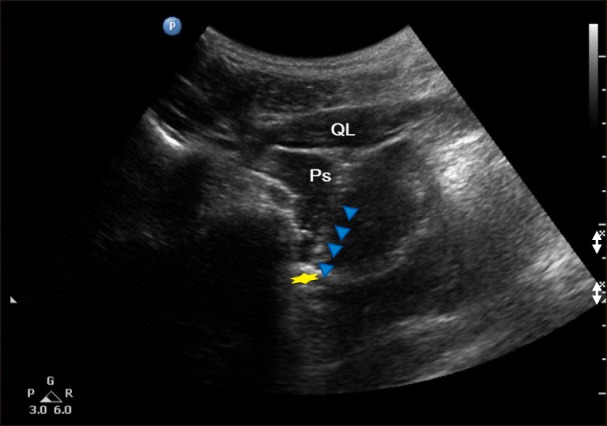
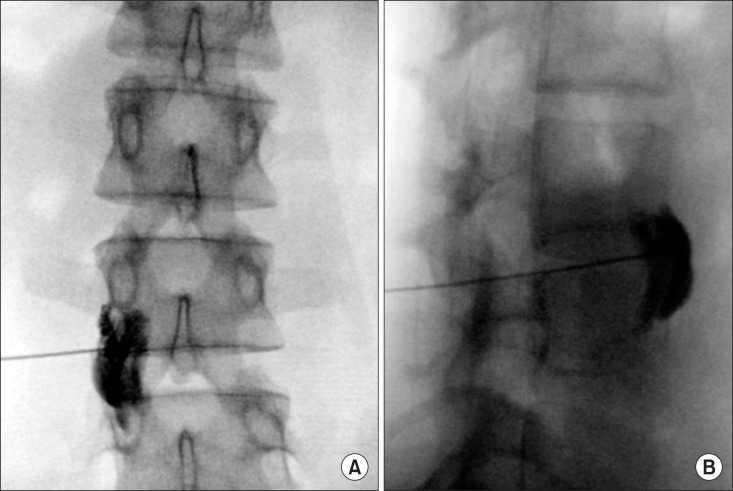
For performing the ultrasound-guided LSB, the target vertebra level (the L3 vertebra was identified by locating the lumbosacral junction (L5-S1 gap; Fig. 1), on paramedian sagittal scanning and counting was done cranially by numbering the lamina and transverse processes of the L5, L4, and L3 vertebrae. After marking the level of the L3 vertebra, the transducer was rotated transversely to obtain a short-axis view showing the transverse process and facet joint. Then, the modified transverse scan of the lumbar paravertebral region through lumbar inter-transverse space was obtained by positioning the transducer at 4-6 cm lateral to the midline in the transverse orientation at the L2-L3 or L3-L4 intervertebral level (Fig. 2). The transducer was also directed medially to insonate the anterior fascia of the psoas major muscle, the target of the needle tip, through the lumbar inter-transverse space. Color Doppler was utilized to determine the presence of vascular structures and to plan needle trajectory. Prior to needle insertion, we visualized the lower pole of kidney, which is usually located approximately at the L3 level, the right kidney being slightly lower than the left. The insertion point of the needle was 8.8 cm lateral to the L3 spinous process. The skin entry point was infiltrated using 1% lidocaine. A curved tip 21 G, 15-cm Chiba needle (Cook Inc., Bloomington, IN, USA) was then advanced toward the anterolateral edge of the target vertebral body using a posterolateral approach. The needle was inserted from a lateral to medial direction using in-plane technique so as to monitor it in real time as it was advanced, using hydrolocalization technique with 1.5 ml of normal saline. The target of the needle tip was the anterior fascia of the psoas major muscle close to the paravertebral space (Fig. 2). After evaluating the location of the needle tip under US and verifying negative aspiration for blood or CSF, 3 ml of contrast dye was administered incrementally to exclude vascular injection by a C-arm image intensifier (Zhiem Vsion R, Ziehm Imaging, Nuernberg, Germany) on the anteroposterior and lateral view, followed by 10 ml of 0.25% levobupivacaine (Fig. 3 and 4). The final depth of the needle was 10.5 cm from the skin.
As an index of the successful LSGB, skin-surface temperatures were monitored with small, adhesive thermocouple probes attached bilaterally to the plantar surface of the feet using transparent patches. The baseline temperature was obtained just before starting the Chiba needle insertion. Temperatures were measured and recorded from the time of the administration of the local anesthetic onwards. The temperature of the left sole rose from 29.3℃ to 33.0℃ over five minutes, an increment of 0.1℃ per 10 seconds. A rise in temperature of as much as 2℃ on the left sole seemed to be an adequate sign of the success of the LSGB [9], therefore, the patient was sent to the recovery room and then was discharged without any adverse events.
An LSGB under a fluoroscope is the most generally used method, due to its convenience and accuracy in confirming the needle tip location or intravascular injection; however, the success rate of fluoroscopic LSGBs has not been satisfactory, ranging from 67% under fluoroscope guidance to 83% under CT-guidance. Furthermore, exposure to radiation during the procedure may be a problem. Manchikanti et al. reports that radiation exposure in seconds raged from 7.4-12.5 seconds during the fluoroscopy guided LSGB [4], and Nordmann et al. [12] estimated the radiation dose during a CT guided LSGB to be between 1.5 and 3.0 mSv. This equates to approximately one year's exposure to natural background radiation in the UK, or the equivalent of about 15-30 chest radiographs. As the patient in this case was a young female, and she needs repetitive LSGBs, it might be a necessary to minimize radiation exposure.
Since the lumbar sympathetic chain is located just anterolateral to the vertebral body, the abdominal aorta and inferior vena cava, located anterior to the vertebral body, are relatively vulnerable to penetration. Because of the poor visualization of the deep tissues and inability to confirm the intravascular injection, the lumbar sympathetic block under ultrasound guidance has only been reported once in 1992 as a new technique [10]. As the research dealt also with CPB, the figures and the description of the technique was focused more on the CPB and was insufficient for the LSGB, and there have not been any following literature about ultrasound-guided LSGBs since. Although US-guided injection with fluoroscopic confirmation has shown similar accuracy and efficacy to fluoroscopy alone for various spinal blocks [5613], no study has sought to compare these techniques for the LSGB, or determine which technique has greater benefits for efficacy and safety during LSGBs; therefore, further study will certainly be needed in this field.
A previous study reported that the lumbar paravertebral space including the psoas muscle, an anatomical key for the LSGB, was visualized on US in approximately two-thirds of the patients [14]. Therefore, we expect that direct visualization of the relative positions of the needle tip in relation to the anterior fascia of the psoas major muscle in the paravertebral space under US-guidance would have similar success rates to fluoroscope-guided LSGBs, along with shortening the procedure time and decreasing procedure-related discomfort which may usually be caused by a needle tip touching the bony cortex of the L3 vertebral body during the fluoroscope-guided LSGB [15]. It is possible, in US-guided LSGBs, to take in real time visualization of the internal organs like the kidney and to easily avoid damage to the internal organs. Therefore we can make the insertion point of needle into the skin more lateral, and reduce the contact pain of the needle striking the bone [16]. Certainly, we need further study to prove the benefits of the US-guided LSGB with fluoroscopic confirmation.
The frequent use of USG for regional anesthesia and pain procedures has familiarized its use among practitioners [1718]. US provides a reliable setup in localizing the anterior fascia of psoas muscle, as well as the relative position of the needle with respect to surrounding tissues. We expect that the US-guided LSGB can be implemented successfully in daily outpatient clinical settings by highly trained pain physicians.
References
1. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001; 99:698–703. PMID: 12022220.
2. Rigaud J, Delavierre D, Sibert L, Labat JJ. Sympathetic nerve block in the management of chronic pelvic and perineal pain. Prog Urol. 2010; 20:1124–1131. PMID: 21056394.
3. Oi Y, Nakamura K, Sakamoto A, Ogawa R, Watari J, Okajima Y, et al. Helical CT guided lumar sympathetic ganglion block. Masui. 1996; 45:888–891. PMID: 8741484.
4. Manchikanti L, Cash KA, Moss TL, Pampati V. Radiation exposure to the physician in interventional pain management. Pain Physician. 2002; 5:385–393. PMID: 16886017.

5. Jee H, Lee JH, Kim J, Park KD, Lee WY, Park Y. Ultrasound-guided selective nerve root block versus fluoroscopy-guided transforaminal block for the treatment of radicular pain in the lower cervical spine: a randomized, blinded, controlled study. Skeletal Radiol. 2013; 42:69–78. PMID: 22609989.

6. Park Y, Lee JH, Park KD, Ahn JK, Park J, Jee H. Ultrasound-guided vs. fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013; 92:575–586. PMID: 23636087.

7. Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep. 2014; 18:424. PMID: 24760493.

8. Gofeld M, Lee CW. Ultrasound-guided superior hypogastric plexus block: a cadaveric feasibility study with fluoroscopic confirmation. Pain Pract. 2016; [in press].

9. Moura RN, De Moura EG, Bernardo WM, Otoch JP, Bustamante FA, Albers DV, et al. Endoscopic-ultrasound versus percutaneous-guided celiac plexus block for chronic pancreatitis pain. A systematic review and meta-analysis. Rev Gastroenterol Peru. 2015; 35:333–341. PMID: 26802887.
10. Kirvelä O, Svedström E, Lundbom N. Ultrasonic guidance of lumbar sympathetic and celiac plexus block: a new technique. Reg Anesth. 1992; 17:43–46. PMID: 1599894.
11. Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007; 8:326–331. PMID: 17610454.

12. Nordmann GR, Lauder GR, Grier DJ. Computed tomography guided lumbar sympathetic block for complex regional pain syndrome in a child: a case report and review. Eur J Pain. 2006; 10:409–412. PMID: 15979912.

13. Finlayson RJ, Etheridge JP, Tiyaprasertkul W, Nelems B, Tran DQ. A randomized comparison between ultrasound- and fluoroscopy-guided c7 medial branch block. Reg Anesth Pain Med. 2015; 40:52–57. PMID: 25478757.

14. Karmakar MK, Ho AM, Li X, Kwok WH, Tsang K, Ngan Kee WD. Ultrasound-guided lumbar plexus block through the acoustic window of the lumbar ultrasound trident. Br J Anaesth. 2008; 100:533–537. PMID: 18344573.

15. Kim DH, Kim KH, Kim YC. Minimally invasive percutaneous spinal techniques. Philadelphia (PA): Elsevier;2011.
16. Kim WH, Kim SK, Lee CJ, Kim TH, Sim WS. Determination of adequate entry angle of lumbar sympathetic ganglion block in Korean. Korean J Pain. 2010; 23:11–17. PMID: 20552067.

17. Rössel T, Kersting S, Heller AR, Koch T. Combination of high-resolution ultrasound-guided perivascular regional anesthesia of the internal carotid artery and intermediate cervical plexus block for carotid surgery. Ultrasound Med Biol. 2013; 39:981–986. PMID: 23499343.

18. Meng S, Lieba-Samal D, Reissig LF, Gruber GM, Brugger PC, Platzgummer H, et al. High-resolution ultrasound of the posterior femoral cutaneous nerve: visualization and initial experience with patients. Skeletal Radiol. 2015; 44:1421–1426. PMID: 26105014.

Fig. 1
Paramedian sagittal image of lumbosacral junction. A target vertebra level is usually identified by locating probe at the lumbosacral junction (L5-S1 gap) and by numbering the lamina and transverse processes upward (blue arrow).

Fig. 2
Modified transverse image of the lumbar paravertebral region through lumbar inter-transverse space. A yellow star shows anterior fascia of the psoas major muscle. PS: psoas major muscle, QL: quadratus lumborum muscle.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download