Abstract
Background
The transforaminal (TF) epidural steroid injection (ESI) is suggested as more effective than the interlaminar (IL) route due to higher delivery of medication at the anterior epidural space. However, serious complications such as spinal cord injury and permanent neural injury have been reported. The purpose of this study is to evaluate and compare the clinical effectiveness, technical ease, and safety of the TF and parasagittal IL (PIL) ESI.
Methods
A total of 72 patients were randomized to either the PIL group (n = 41) or the TF group (n = 31) under fluoroscopic guidance. Patients were evaluated for effective pain relief by the numerical rating scale (NRS) and Oswestry Disability Index (ODI) (%) before and 2 weeks after the ESI. The presence of concordant paresthesia, anterior epidural spread, total procedure time, and exposed radiation dose were also evaluated.
Results
Both the PIL and TF approach produced similar clinically significant improvements in pain and level of disability. Among the 72 patients, 27 PIL (66%) and 20 TF (64%) patients showed concordant paresthesia while 14 (34%) and 11 (36%) patients in the same respective order showed disconcordant or no paresthesia. Radiation dose and total procedure time required were compared; the PIL group showed a significantly lower radiation dose (30.2 ± 12 vs. 80.8 ± 26.8 [Cgy/cm2]) and shorter procedure time (96.2 ± 31 vs. 141.6 ± 30 seconds).
Chronic low back pain with or without radicular leg pain is the most common form of chronic pain, and is an important social, clinical and public health problem [1]. Various simple conservative and surgical treatments are available, although conservative options with minimal interventions are now favored in the wake of unsatisfactory surgical outcomes with high surgical costs [234].
Epidural steroid injection (ESI) via the transforaminal (TF) or interlaminar (IL) approach is a commonly performed procedure to improve chronic low back pain with lower extremity pain in patients with spinal stenosis or intervertebral disc disease. ESI improves pain by reducing the inflammatory cascade either by inhibiting the synthesis or release of pro-inflammatory substances [4567].
TF and IL are the two main approaches of ESI. Many pain physicians prefer TF injections due to the advantage of delivery of a high concentration of medication to the anterior epidural space where various pain substances such as substance P and glutamate exist [89]. ESI could provide the most effective pain relief if the medications are delivered close to the site of the pathology [1011]. The TF route has been associated with many complications including paraplegia, spinal cord injury, permanent paralysis, intradiscal injection of the medication, and even death [1213141516]. Therefore, the safety issue of the TF route has emerged and various efforts to come up with a technically easy and better route with fewer complication are essential. ESI via the IL approach is technically less challenging and has been widely used, but its clinical outcome is reportedly limited [4511]. The main reason of this poor outcome is thought to be the delivery of medication to the dorsal epidural space with limited ventral epidural spread. However, recent studies suggested that parasagittal IL (PIL) ESI with the needle located in the most lateral part of the interlaminar space resulted in 89-100% of ventral spread of contrast dye in contrast to the 31.7% ventral spread by midline IL ESI [111718]. PIL ESI has demonstrated superior outcomes in terms of the visual analogue scale (VAS) and Oswestry Disability Index (ODI) compared to midline IL ESI [11].
The clinical efficacy of ESI according to the different approaches varies and it is difficult to conclude definitely that one method is superior to another. Gupta et al. [19] compared the midline, parasagittal, and TF approaches, and concluded that the TF approach is better in terms of VAS reduction than midline and parasagittal injections. However, Ghai et al. [10] compared TF and PIL ESI, and demonstrated a similar rate of effective pain relief, pain relief survival period, ventral epidural spread, and fluoroscopy time.
We have observed in our clinical practice that PIL ESI provides equivalent pain relief in terms of the VAS and ODI compared to TF ESI with greater technical ease. The main purpose of this study was to evaluate and compare the clinical effectiveness and identify the method that is technically easier and the safer route.
This study was a prospective, single center, randomized and blinded study, conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines. This study was approved by the institutional review board of our institution (05-029) and all participants provided written and informed consent. This trial was registered in the Clinical Trial Registry (NCT02838615).
Eighty patients who received fluoroscopically guided TF ESI or PIL ESI from April 2016 to January 2017 were enrolled in this study. These patients had chronic low back pain and unilateral radicular pain due to intervertebral disc herniation. They did not show any response to medication and physical therapy during a 3-month period of conservative therapy. We also confined the included patient group to patients having a pain and disability score of at least 5 and 30% as assessed on the 0–10 numerical rating scale and 0–100% ODI at baseline, respectively. Patients were evaluated clinically using history taking and a straight leg raise test, and final diagnosis of intervertebral disc protrusion or extrusion was confirmed after checking magnetic resonance imaging (MRI).
Patients were excluded if they had any laboratory findings suggesting coagulopathy, inflammation, or infection; allergy to contrast dye, steroids, or local anesthetics; previous surgery on the lumbar spine; and unstable neurologic deficits, or cauda equine syndrome. Patients who had received ESI in the previous 6 months were also excluded. Patients who had stopped taking anticoagulants for the proper time before ESI were included in this study.
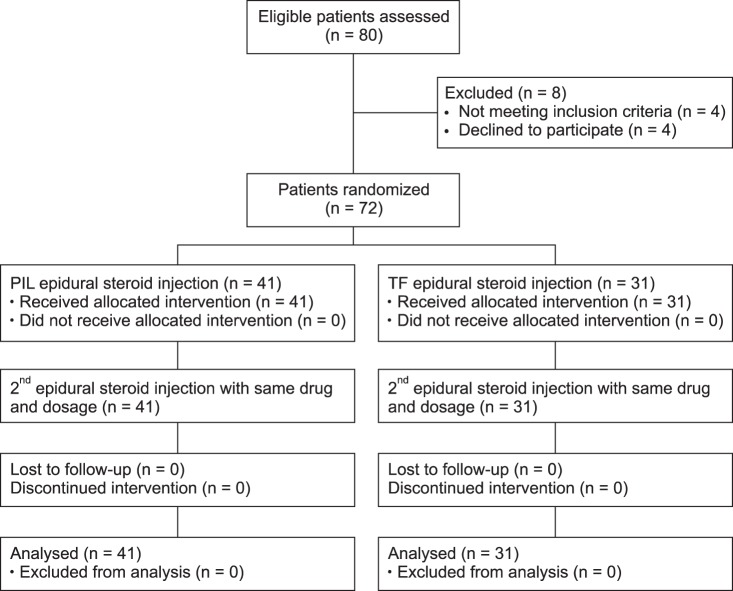
Eighty patients were enrolled in this study and 8 patients were excluded due to not satisfying the inclusion criteria and refusal to participate in this study. Finally, 72 patients were enrolled and 72 cases of TF ESI and PIL ESI were evaluated.
Patients were randomized to receive ESI either by the TF or PIL approach. Randomization was performed using a computer-generated randomization table. Random numbers were kept in sealed envelopes and opened by an independent clinical research nurse at the time of injection. None of the investigators involved in this study had any access to the randomization table. All ESIs were performed by a single investigator (JH) who had over 10 year experiences in fluoroscopically guided injection.
All clinical data were obtained before the ESI, and these included age, gender, duration of symptoms, level of disc herniation, and degree of depression. Depressive symptoms were evaluated using the Korean version of the Beck Depression Inventory (BDI). BDI is a 21-item, self-reported questionnaire, which can evaluate somatic, affective, and cognitive symptoms of depression [20]. If a patient had any difficulty in understanding some items of the questionnaire, a clinical research nurse explained such items to help their understanding. We encouraged all patients to self-complete the BDI questionnaire.
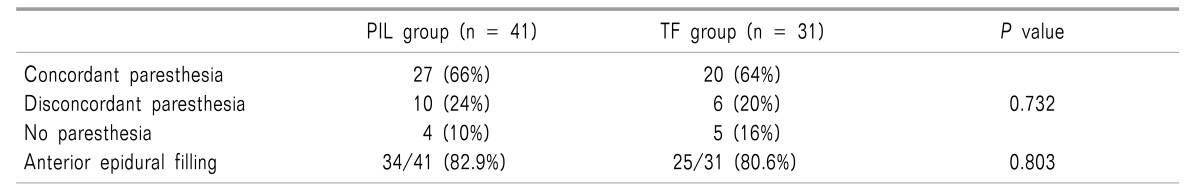
We also obtained data about anterior epidural spreading and the presence of concordant paresthesia during TF or PIL ESI to identify the actual incidence. To confirm the anterior epidural spread, we evaluated the epidural spread pattern using the lateral view of a fluoroscopic image after injecting 3 ml of contrast dye. The presence of concordant paresthesia was evaluated by asking the patients directly after injection of the mixture of dexamethasone and local anesthetics. Patients were asked if the pain was felt in the similar distribution or direction as their original pain (concordant), was dissimilar, or was absent in both quality and location.
We measured the total procedure time required to complete TF or PIL ESI using a stop watch (Dretec, Japan) to assess the technical ease between the two methods. The measured time was from skin infiltration of lidocaine until the end of the injection of contrast dye to confirm successful TF or PIL ESI. We also measured the amount of radiation (cGy/cm2) exposure during the same period of the total procedure and analyzed the total amount of radiation exposure through the recorded value that was automatically saved in the C-arm. During these measurements, if the TF or PIL ESI was unsuccessful by incidental vascular or intradiscal puncture, measurement was performed once again at the second TF or PIL ESI.
A numerical rating scale (NRS, 0; no pain, 10; worst pain imaginable) and the Korean version of the ODI (0–50) was used to evaluate the initial clinical status in terms of degree of pain and disability level. TF or PIL ESI was done twice with a 2-week interval between sessions before the final treatment outcome evaluation with the NRS and ODI. All patients self-reported their average severity of pain symptoms over the prior week.
The second ESI of the same type was performed 2 weeks after the initial ESI. A pain physician who was blinded to the type of ESI reevaluated each patient using the NRS and ODI 14 days after the second ESI. Before obtaining the follow-up evaluation data including the NRS and ODI, we explained the initial NRS and ODI scores, which were self-reported during the pre-injection period and helped them assess their severity of pain and disability level more clearly.
One pain physician with more than 10 years of experience with both techniques performed all ESIs in the same fashion. This minimized technical variability that would have occurred if several pain physicians had been used. The pain physician performing every procedures was blinded to all data related to clinical outcome. Decisions concerning the level and side of injection were made based according to the presenting symptoms of the patient and the level of disc herniation confirmed by MRI.
The patient was positioned prone with a pillow under the lower abdomen to minimize the lumbar lordosis and draped in a sterile fashion. The desired interlaminar epidural space was confirmed using anteroposterior (AP) imaging. The superior border of the inferior lamina was marked and the skin with subcutaneous tissue overlying the target point was infiltrated with 1% lidocaine using a 25-gauge, 1.5-inch needle. A 21-gauge Tuohy needle (Taechang Industrial Co., Kongju, Korea) was inserted under AP fluoroscopic guidance until the needle reached the superior border of the inferior lamina. If the bony contact was made with the superior border of the inferior lamina, loss of resistance technique was used to verify entry into the epidural space.
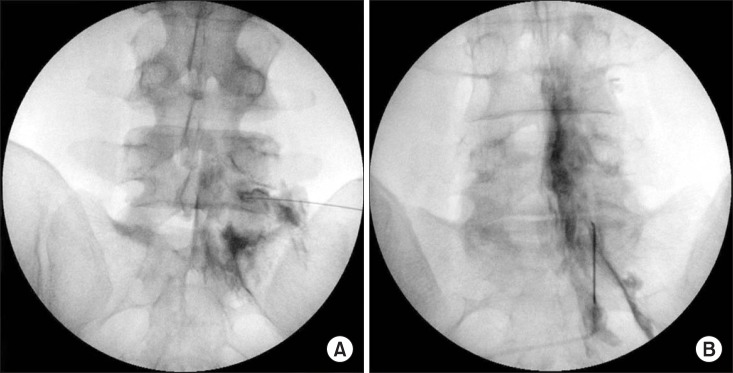
When loss of resistance with air was felt during an advance under AP fluoroscopic guidance, a lateral fluoroscopic image was obtained to confirm that the needle was located within the posterior border of the spinal canal. After checking the final needle position, 3 ml of contrast media was injected and the epidural injection was confirmed using an AP and lateral fluoroscopic image. The presence of anterior epidural spread was evaluated using a lateral view only in the cases where successful epidural contrast spread was obtained.
A mixture of 5.0 mg dexamethasone and 3 ml 0.2% ropivacaine was injected as a therapeutic medication just after evaluation of the epidural contrast spread pattern. After completion of this injection, the patient was asked if they felt any pain during the injection and if the experienced pain was similar or dissimilar to their original pain.
The patient was positioned prone with a pillow under the lower abdomen to minimize the lumbar lordosis and draped in a sterile fashion. The desired spinal level was confirmed using AP imaging and the inferior endplate of the desired level was modulated to the linear line by tilting the C-arm into a cephalad or caudal direction. A 25-gauge Whitacre spinal needle was advanced under intermittent fluoroscopic guidance with an oblique view toward the 6 o'clock position of the pedicle. AP and lateral fluoroscopic images were used during the advancement of the needle toward the intervertebral foramen and superolateral to the exiting spinal nerve. Special care was taken to minimize the risk of intravascular and disc puncture.
After checking the final needle position, 3 ml of contrast media was injected, and the epidural injection was confirmed using AP and lateral fluoroscopic images. The presence of anterior epidural spread was evaluated using a lateral view in cases of successful epidural contrast spread. A mixture of 5.0 mg dexamethasone and 3 ml 0.2% ropivacaine was injected as a therapeutic medication just after evaluation of the epidural contrast spread pattern. After completion of this injection, the patient was asked if they felt any pain during the injection and if the experienced pain was similar or dissimilar to their original pain.
The independent Student's t-test was used to compare the continuous variables of treatment outcome (NRS, and ODI [%]), baseline demographic characteristics (age, duration of pain, BDI, NRS and ODI [%]) and radiation dose, as well as the total procedure time required for PIL or TF ESI. The chi square test was used to analyze the incidence of concordant paresthesia and anterior epidural spreading.
According to our preliminary study, the incidence of ventral epidural spread in the PIL ESI was 90% while 60% in the TF ESI. Therefore, assuming the difference of incidence rate as 30%, and the α error as 0.05 and β error as 0.2 with 80% power, 31 patients were required in each group. All statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). The results were considered statistically significant if the P value was less than 0.05.
The patient flow diagram is illustrated in Fig. 1. The fluoroscopic image of the TF and PIL ESI is shown in Fig. 2.
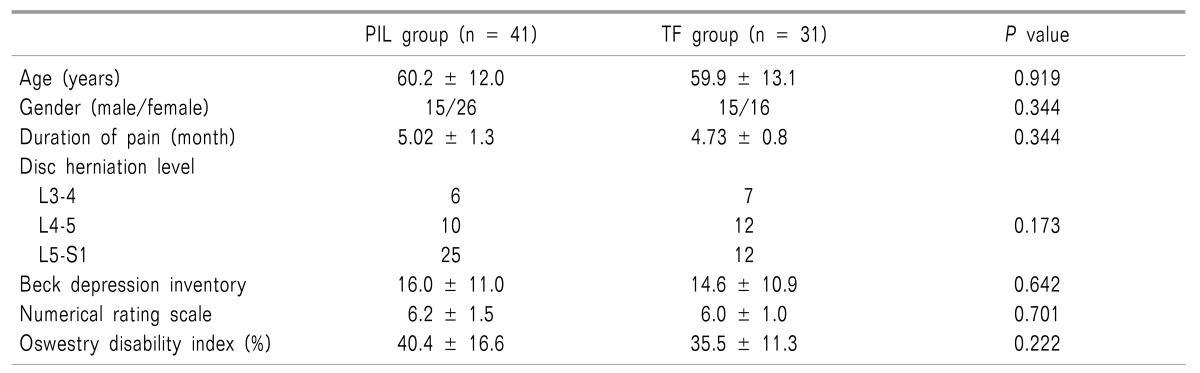
Seventy-two patients were enrolled; 41 were randomized to TF ESI and 31 to PIL ESI. As a baseline demographic data, the disc herniation level (L3-4, L4-5, L5-S1) was most frequently at L5-S1 (Table 1). If a patient had disc herniation at more than one level, the severity of the protruded disc level was assessed using MRI and the disc level with the greater protrusion was recorded as the level of disc herniation. The initial BDI, NRS, and ODI (%) scores were similar between the PIL and TF groups (Table 1).
Clinical outcomes were evaluated using the NRS and ODI (%) which were obtained at baseline (pretreatment) and 2 weeks after the final ESI. There were no significant differences in the NRS and ODI (%) when compared between the PIL and TF groups. The NRS and ODI (%) were significantly reduced after 2 weeks in both groups (P < 0.001) (Table 2). Overall, both the IL and TF approach produced similar clinically significant improvements in pain and level of disability.
Among the 72 patients treated with PIL or TF ESI, 27 (66%) and 20 (64%) patients, respectively, showed concordant paresthesia, while 14 (34%) and 11 (36%), respectively showed disconcordant or no paresthesia. The number of patients with anterior epidural spreading was similar between the PIL and TF groups (Table 3).
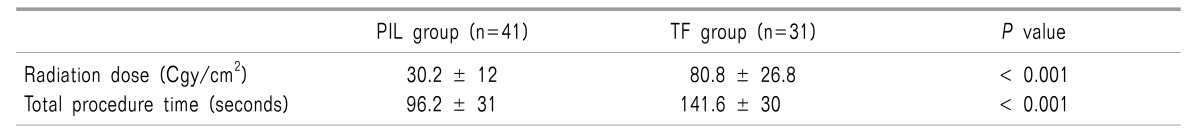
Radiation dose and total procedure time required for PIL and TF group were compared. The PIL group showed a significantly less radiation dose (30.2 ± 12 vs. 80.8 ± 26.8 [Cgy/cm2]) and shorter procedure time (96.2 ± 31 vs. 141.6 ± 30 seconds) (Table 4).
Any potential complications of ESI were not found for both PIL and TF approaches.
According to this study, PIL ESI provided equivalent pain relief in terms of the VAS and ODI reduction compared to TF ESI with a significant shorter time required to perform ESI and a lower dose of radiation exposure. Previous studies also suggested that PIL ESI demonstrated equivalent or superior efficacy compared to TF ESI or ESI with a midline approach [91011]. However, Gupta et al. [19] reported that TF ESI resulted in better clinical outcomes in terms of number of patients who had more than 50% relief than the midline and parasagittal approaches. The latter study demonstrated clinical efficacy by evaluating the number of patients who showed more than 50% improvement. The present study assessed clinical efficacy by comparing the mean VAS and ODI (%) reduction, therefore, direct comparison between the two studies is limited. In addition, PIL ESI and TF ESI displayed equivalent clinical efficacy for the first month following each procedure [19]. Why more patients in the TF group displayed improved clinical efficacy at 3 months compared to 1 month warrants further study.
Most disc herniations are located between the posterior vertebral body and the anterior to ventral epidural space. Therefore, if an epidural steroid is injected into the anterior epidural space, which is abundant in pain substance due to the irritated disc material, the clinical effect after ESI would be maximized [21]. The presence of anterior epidural spread of the medication showed a definite correlation with clinical improvement [111922]. There are several suggested technical reasons why PIL ESI resulted in equivalent or similar clinical outcomes with TF ESI. PIL ESI targets the far lateral side of the interlaminar space, which is close to the anterior epidural space. In addition, due to the proximity to the anterior epidural space, it is easier to deliver the medication around the irritated nerve root. We suppose that properly delivered medication into the anterior epidural space and around the irritated nerve root by a PIL approach provided the equivalent therapeutic effect.
The incidence of anterior epidural and perineural spread during PIL ESI was reported to be 70–100% and 20–62%, respectively [9111719]. The present finding of an 82.9% incidence of anterior epidural spread in the PIL group is similar to previous studies. Interestingly, Candido et al. [17] reported 100% and 75% rates of anterior epidural placement of contrast media in the PIL and TF groups, respectively, and concluded that the PIL approach better promotes anterior epidural spread.
Concordant paresthesia is a subjective painful sensation which is felt during ESI matching the distribution of the original painful side, whereas disconcordant pain is dissimilar in quality and distribution. The reappearance of previous daily and typical pain during a lumbar ESI may indicate proper delivery of medication to the target, thus increasing the chance of improved pain resolution and decreased disability. Previous studies suggested that concordant paresthesia or pain provocation during lumbar ESI correlates well with pain relief and is a good prognostic indicator [182324]. In this study, we evaluated the incidence of concordant paresthesia and both groups showed more than 60% concordant paresthesia. The incidence of concordant paresthesia in prior studies ranged from 44–81% and foraminal stenosis, nerve root impingement, and lack of a medial-superior contrast flow pattern were associated with concordant pain provocation during TF ESI [182325].
In this study, we checked the exposed radiation dose and total procedure time required to perform TF ESI and PIL ESI to assess the technical ease of both procedures. The exposed radiation dose and total procedure time of TF group was 3 and 1.5 times higher than that of PIL group, respectively. For the pain intervention, the use of fluoroscopy is essential to ensure the accuracy of therapeutic injections. In our pain practice, we perform pain interventions under fluoroscopic guidance in at least 70% of all interventions. The increase in fluoroscopy-guided ESI has led to a growing concern with radiation exposure and radiation dose. Radiation exposure was dependent on the practitioners and the methods of lumbar ESI [26].
There might be slight variations among individual practices in almost every technical approach of ESI. In our clinical practice, we perform PIL ESI only under AP fluoroscopic guidance until the needle touches the superior border of the inferior lamina. If bony contact is made with the superior border of the inferior lamina, then, loss of resistance is used to verify entry into the epidural space. Before the bony contact was made, we rarely rotated the C-arm to obtain the lateral view, and intermittently 2 to 3 AP fluoroscopic views were enough to ensure that the epidural needle had reached the superior border of the inferior lamina. We did not use the lateral view during advancement to the inferior lamina because before the needle touches the lamina, only subcutaneous fat and muscles are located in the needle's path. The ligamentum flavum, to which we should pay more attention, exists on the inner side of lamina [27].
However, after contacting the inferior lamina and redirecting the needle to the interlaminar area, loss of resistance combined with the guidance of the lateral view should help prevent inadvertent dural puncture. No patient suffered from a dural puncture headache due to inadvertent dural puncture in either group. Using this method during PIL ESI allowed us to reduce the time and radiation dose exposure compared to the TF group. However, in the TF approach, continuous AP and lateral fluoroscopic views should be obtained during advancement of the needle.
Our results support the superiority of PIL ESI compared to TF ESI considering the equivalent clinical outcome in terms of the NRS and ODI (%) reduction, and the greater technically ease of PIL, that was evident in the shorter procedure time with a lower dose of radiation exposure compared to the TF approach. Safety concerns of TF ESI include paraplegia, quadriparesis, and that intradiscal injections also bolster the use of PIL [131415].
Our study has several limitations. The clinical outcome was evaluated 2 weeks after the final ESI and no additional long term follow-up was made. However, we focused on the short term therapeutic effect of ESI and so that we could restrict potential factors, such as medications and physical therapy types after ESI, which could affect the clinical outcomes of the ESIs. Also, we could minimize the number of patients who failed to return for the outcome evaluation. During the injection of medication, we assessed the presence of concordant or disconcordant paresthesia. However, we encountered difficult cases of assessment due to ambiguous patient expression of the sensations they experienced. In such cases, we reevaluated the paresthesia sensation after encouraging and teaching patients to express their sensation more objectively.
ESI under fluoroscopic guidance with a PIL or TF approach were both effective in reducing the NRS and ODI (%), but PIL ESI was a technically easier and simpler method. The incidence of concordant paresthesia and anterior epidural spread, which were good prognostic indicators, was similar between the two groups [182324]. Potential complications of ESI were not found for either group.
References
1. Manchikanti L, Buenaventura RM, Manchikanti KN, Ruan X, Gupta S, Smith HS, et al. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician. 2012; 15:E199–E245. PMID: 22622912.
2. Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine (Phila Pa 1976). 2009; 34:1094–1109. PMID: 19363455.
3. Carreon LY, Glassman SD, Howard J. Fusion and non-surgical treatment for symptomatic lumbar degenerative disease: a systematic review of Oswestry Disability Index and MOS Short Form-36 outcomes. Spine J. 2008; 8:747–755. PMID: 18037354.

4. Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: a comprehensive, evidence-based review. Reg Anesth Pain Med. 2013; 38:175–200. PMID: 23598728.
5. Manchikanti L, Knezevic NN, Boswell MV, Kaye AD, Hirsch JA. Epidural injections for lumbar radiculopathy and spinal stenosis: a comparative systematic review and meta-analysis. Pain Physician. 2016; 19:E365–E410. PMID: 27008296.
6. Manchikanti L, Singh V, Pampati V, Falco FJ, Hirsch JA. Comparison of the efficacy of caudal, interlaminar, and transforaminal epidural injections in managing lumbar disc herniation: is one method superior to the other? Korean J Pain. 2015; 28:11–21. PMID: 25589942.

7. Manchikanti L, Cash KA, Pampati V, Falco FJ. Transforaminal epidural injections in chronic lumbar disc herniation: a randomized, double-blind, active-control trial. Pain Physician. 2014; 17:E489–E501. PMID: 25054399.
8. Kraiwattanapong C, Wechmongkolgorn S, Chatriyanuyok B, Woratanarat P, Udomsubpayakul U, Chanplakorn P, et al. Outcomes of fluoroscopically guided lumbar transforaminal epidural steroid injections in degenerative lumbar spondylolisthesis patients. Asian Spine J. 2014; 8:119–128. PMID: 24761192.

9. Gharibo CG, Varlotta GP, Rhame EE, Liu EC, Bendo JA, Perloff MD. Interlaminar versus transforaminal epidural steroids for the treatment of subacute lumbar radicular pain: a randomized, blinded, prospective outcome study. Pain Physician. 2011; 14:499–511. PMID: 22086091.
10. Ghai B, Bansal D, Kay JP, Vadaje KS, Wig J. Transforaminal versus parasagittal interlaminar epidural steroid injection in low back pain with radicular pain: a randomized, double-blind, active-control trial. Pain Physician. 2014; 17:277–290. PMID: 25054387.
11. Ghai B, Vadaje KS, Wig J, Dhillon MS. Lateral parasagittal versus midline interlaminar lumbar epidural steroid injection for management of low back pain with lumbosacral radicular pain: a double-blind, randomized study. Anesth Analg. 2013; 117:219–227. PMID: 23632053.

12. Kennedy DJ, Dreyfuss P, Aprill CN, Bogduk N. Paraplegia following image-guided transforaminal lumbar spine epidural steroid injection: two case reports. Pain Med. 2009; 10:1389–1394. PMID: 19863744.

13. Candido KD, Katz JA, Chinthagada M, McCarthy RA, Knezevic NN. Incidence of intradiscal injection during lumbar fluoroscopically guided transforaminal and interlaminar epidural steroid injections. Anesth Analg. 2010; 110:1464–1467. PMID: 20418306.

14. Thefenne L, Dubecq C, Zing E, Rogez D, Soula M, Escobar E, et al. A rare case of paraplegia complicating a lumbar epidural infiltration. Ann Phys Rehabil Med. 2010; 53:575–583. PMID: 20870478.

15. Lyders EM, Morris PP. A case of spinal cord infarction following lumbar transforaminal epidural steroid injection: MR imaging and angiographic findings. AJNR Am J Neuroradiol. 2009; 30:1691–1693. PMID: 19369604.

16. Levi D, Horn S, Corcoran S. The incidence of intradiscal, intrathecal, and intravascular flow during the performance of retrodiscal (infraneural) approach for lumbar transforaminal epidural steroid injections. Pain Med. 2016; 17:1416–1422. PMID: 26814293.

17. Candido KD, Raghavendra MS, Chinthagada M, Badiee S, Trepashko DW. A prospective evaluation of iodinated contrast flow patterns with fluoroscopically guided lumbar epidural steroid injections: the lateral parasagittal interlaminar epidural approach versus the transforaminal epidural approach. Anesth Analg. 2008; 106:638–644. PMID: 18227326.

18. Hong J, Jung S. Clinical effectiveness and prognostic indicators of parasagittal interlaminar epidural injection. Pain Physician. 2016; 19:E877–E884. PMID: 27454278.
19. Gupta R, Singh S, Kaur S, Singh K, Aujla K. Correlation between epidurographic contrast flow patterns and clinical effectiveness in chronic lumbar discogenic radicular pain treated with epidural steroid injections via different approaches. Korean J Pain. 2014; 27:353–359. PMID: 25317285.

20. Jo SA, Park MH, Jo I, Ryu SH, Han C. Usefulness of Beck Depression Inventory (BDI) in the Korean elderly population. Int J Geriatr Psychiatry. 2007; 22:218–223. PMID: 17044132.

21. Botwin KP, Natalicchio J, Hanna A. Fluoroscopic guided lumbar interlaminar epidural injections: a prospective evaluation of epidurography contrast patterns and anatomical review of the epidural space. Pain Physician. 2004; 7:77–80. PMID: 16868616.
22. Hashemi M, Mofrad MK, Mohajerani SA, Kazemi SM, Radpey B, Zali A. Anatomical flow pattern of contrast in lumbar epidural space: a human study with a midline vs. parasagittal interlaminar approach under fluoroscopy. Pain Physician. 2015; 18:317–324. PMID: 26218934.
23. Candido KD, Rana MV, Sauer R, Chupatanakul L, Tharian A, Vasic V, et al. Concordant pressure paresthesia during interlaminar lumbar epidural steroid injections correlates with pain relief in patients with unilateral radicular pain. Pain Physician. 2013; 16:497–511. PMID: 24077196.
24. Sinofsky AH, Aydin SM, Kim E, Gharibo CG. Concordant provocation as a prognostic indicator during interlaminar lumbosacral epidural steroid injections. Pain Physician. 2014; 17:247–253. PMID: 24850106.
25. McCormick Z, Margolis S, Temme K, Rivers E, Cameron SA, Smith MC, et al. Concordant pain provocation during transforaminal epidural steroid injection for lumbosacral radiculopathy: effect on pain outcome and predictive factors. Pain Physician. 2015; 18:E19–E26. PMID: 25675066.
26. Kim S, Shin JH, Lee JW, Kang HS, Lee GY, Ahn JM. Factors affecting radiation exposure during lumbar epidural steroid injection: a prospective study in 759 patients. Korean J Radiol. 2016; 17:405–412. PMID: 27134528.

27. Derby R, Melnik I, Choi J, Lee SH, Lee JE. Reliability and safety of contra-lateral oblique view for interlaminar epidural needle placement. Pain Physician. 2017; 20:E65–E73. PMID: 28072798.
Table 2
Changes of Numerical Rating Scale and Oswestry Disability Index (%) after Parasagittal Interlaminar (PIL) or Transforaminal (TF) Epidural Injection
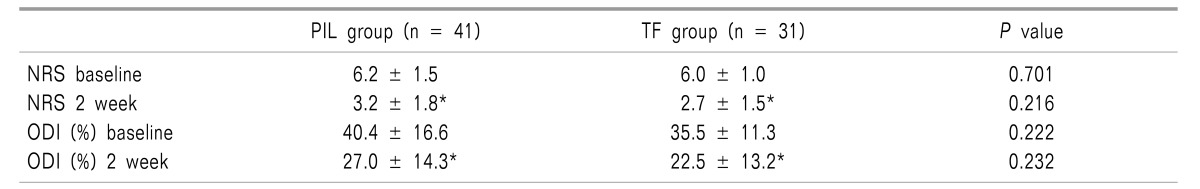
Values are mean ± SD. NRS: numerical rating scale, ODI (%): oswestry disability index. There were no significant differences in NRS and ODI (%) at baseline and 2 weeks after epidural injection between PIL and TF group. However, the scores of NRS and ODI (%) at baseline significantly reduced at 2 weeks after ESI in both groups *(P < 0.001).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download