Abstract
Background
Surgeon satisfaction and patient analgesia during the procedure of laparoscopic surgery are important issues. The aim of this work was to study if an intrathecal (IT) Bupivacaine combined with Magnesium sulfate may or may not provide good surgeon satisfaction in addition to improvement of intraoperative and postoperative analgesia.
Methods
Sixty female patients were enrolled in this prospective, randomized, double-blind controlled clinical trial study. All patients were operated for gynecological laparoscopic surgery under spinal anesthesia. Patients were divided into two groups (Bupivacaine and Magnesium). Group Bupivacaine (30 patients) received intrathecal Bupivacaine 0.5% only (15 mg), while 30 patients in group Magnesium received intrathecal Bupivacaine (15 mg) in addition to intrathecal Magnesium sulfate (50 mg). The sensory block level, the intensity of motor block, the surgeon satisfaction, the intraoperative visual analog scale (VAS) for pain assessment, the postoperative VAS, and side effects were recorded during the intraoperative period and within the first 24 hours after surgery in the post-anesthesia care unit.
Results
Surgeon satisfaction, intraoperative shoulder pain, postoperative pain after 2 h, and perioperative analgesic consumption (ketorolac) were significant better in group Magnesium than in group Bupivacaine. (P < 0.05). The onset of motor and sensory blocks was significant longer in group Magnesium than the other one. The incidence of PONV, pruritus and urinary retention was insignificant statistically between both groups.
Go to : 
The laparoscopic surgery is now a widely established procedure. Benefits of laparoscopy include less postoperative pain, a better cosmetic result, and better patient satisfaction with early discharge. Many surgical procedures are routinely performed using laparoscopy including urological, gastrointestinal, and gynecological techniques. There is an increase in the number of elective and emergency operations performed laparoscopically. Most patients undergoing gynecological procedures are young and fit. Laparoscopic surgery includes insufflation of a carbon dioxide to produce pneumoperitoneum. Laparoscopic surgery is usually done under general anesthesia. Regional anesthesia techniques can also be used for laparoscopic procedures [12].
The advantages of general anesthesia over spinal anesthesia include good control of both airways and ventilation, and promotion of muscular relaxation, making the field optimal for the surgeon to do the procedure, so many anesthesiologists and surgeons prefer it. Insufflation of carbon dioxide increases the incidence of discomfort and chances of neck and shoulder pain, especially when the procedure was done under regional anesthesia in which patients are awake, and cannot tolerate the adverse effects from the pneumoperitoneum [123].
However, some researchers have demonstrated the safety and efficacy of gynecological laparoscopic surgery under spinal anesthesia [4567]. The hypothesis of this study was that adding Magnesium sulfate to intrathecal Bupivacaine might improve surgeon satisfaction and analgesia during and after laparoscopic gynecological surgeries.
Go to : 
This prospective, randomized, double-blind clinical trial was performed after local ethics committee approval was received from the faculty of medicine of Assiut University (Ref: IRB000087110) and registered with ClinicalTrials.gov (ref: NCT02671227). A written informed consent was obtained from all participants. Sixty female patients with American Society of Anesthesiologists (ASA) physical status I or II aged 20-50 yrs., who underwent elective laparoscopy using gynecological techniques were included.
Exclusion criteria included use of steroids or antiemetics, as well as patients with liver, pulmonary, cardiac or renal diseases, pregnancy, or BMI > 30 kg/m2, previous surgeries, allergy to Bupivacaine or Magnesium sulfate, and known neuromuscular disorders. All patients were required to provide a negative pregnancy test within the last 24 hours before surgery.
Patients were allocated randomly by a computer-generated randomization sequence into two equal groups (Bupivacaine and Magnesium). Patients in the Bupivacaine group received intrathecal Bupivacaine 0.5% only (15 mg), while those in the Magnesium group received intrathecal Bupivacaine (15 mg) in addition to intrathecal Magnesium (50 mg).
Full preoperative anesthetic assessments for cardiovascular problems or respiratory diseases were carried out because of the adverse effects of the insufflation of CO2 and the Trendelenburg position. Premedication was used only in anxious patients. Electrocardiogram, non-invasive arterial blood pressure, and arterial oxygen saturation monitors were connected and continuously monitored during the operation.
A subarachnoid block was performed with the patient in the sitting position using the sterile technique at the L3-4 interspace. All patients received intrathecal 15 mg hyperbaric Bupivacaine (0.5%) in the Bupivacaine group, with the addition of Magnesium sulfate (50 mg) in the Magnesium group.
Intrathecal drugs were injected over 10 seconds with the needle orifice directed to the cephalad. The patient was placed in a supine position. Intravenous fluids were administered at the anesthesiologist's discretion as per usual practice in patients in both groups (10 ml/kg of lactated Ringer solutions) over the duration of the operation. Oxygen was delivered to the patients by face mask at 3L/minute. The level of the subarachnoid block was assessed using the loss of cold sensation to ice, measured every two minutes after spinal anesthesia. Once the T6 block was established, the surgery was allowed to proceed.
Carbon dioxide is insufflated into the peritoneal cavity at a rate of 4-6 L/minutes to a pressure of 10-20 mm Hg. The pneumoperitoneum is maintained by a constant gas flow of 200-400 ml/min. The patients were positioned in Trendelenburg position. The surgical laparoscopic technique was performed. After the end of the surgery, the patients were repositioned in the reverse Trendelenburg position.
Intraoperative hypotension was defined as a more than 30% fall in mean blood pressure, and was managed with intravenous ephedrine (6 mg). Intraoperative bradycardia (defined as pulse rate < 60 Bpm) was controlled with intravenous atropine (0.5 mg).
Surgeon satisfaction (scale from 1 to 10) was assessed intraoperatively (from the beginning of the gynecologic laparoscopic procedure till the end of the process) as a primary outcome. Shoulder-tip pain has been evaluated using the Visual Analog Scale (VAS) during the intra-operative period. A nurse, who was unaware of the groups to which the patient belonged, evaluated patients at 0, 6, 12, and 24 h after surgery for any symptoms of pain using the VAS.
Ketorolac 30 mg (IV) was administered on patient request or when the VAS score was > 3. The total use of rescue analgesia in the first postoperative 24 h was recorded. The onset, time to reach the maximum height, and duration of the sensory block were tested with the pinprick method. Demographic parameters, pulse rate per min, and mean arterial blood pressure (MBP) were recorded preoperatively, at 10 min, 30 min, and 150 min. Various side effects like the incidence of nausea-vomiting, pruritus, and shivering were recorded.
Thirty patients in each group were needed to test a 20% difference in the proportions of surgeon satisfaction with an (80%) power of study and an α level of 0.05. Data were analyzed using the Statistical Package for Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA).
Normality of data distribution was evaluated with the Kolmogorov-Smirnov test. Outcome variables were presented in the form of mean ± SD, median (interquartile range) or number (%). Statistical analysis utilized the independent samples t-test or Mann-Whitney test for continuous variables, and the Chi-square and Fisher's exact tests for categorical variables. The significance level was P < 0.05.
Go to : 
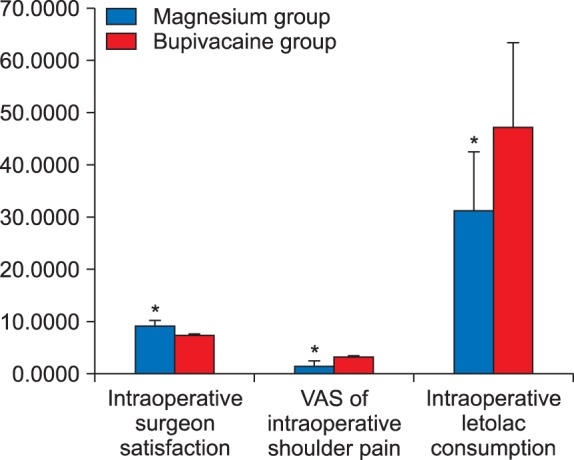
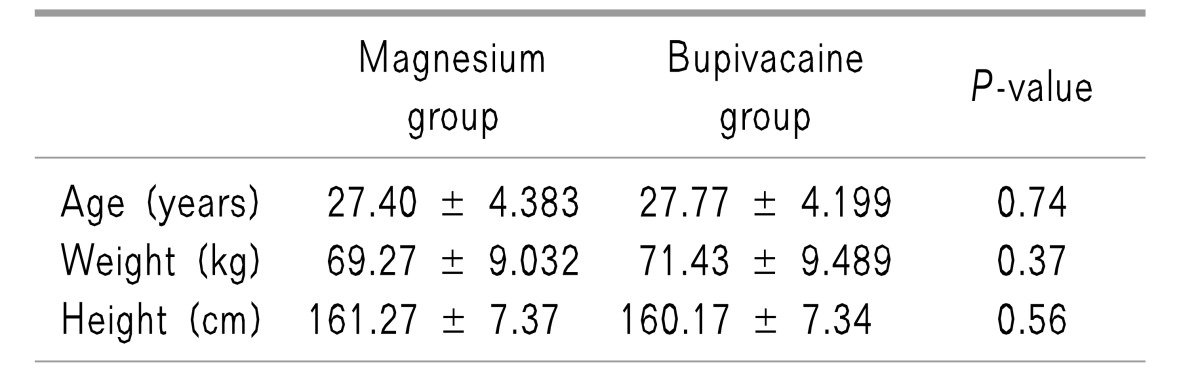
All patients completed the study, with 30 patients in the Magnesium group and 30 in the Bupivacaine group. The patients' characteristics, including age, height, and weight, were all similar in both groups with statistically insignificant differences between them (Table 1). Surgeon satisfaction was significantly better in the Magnesium group [9 (1.25)] than in the Bupivacaine group [7 (2)], (P < 0.001), (Fig. 1). Intraoperative shoulder pain was less in the Magnesium group than in the Bupivacaine group, (P < 0.001), (Fig. 2).
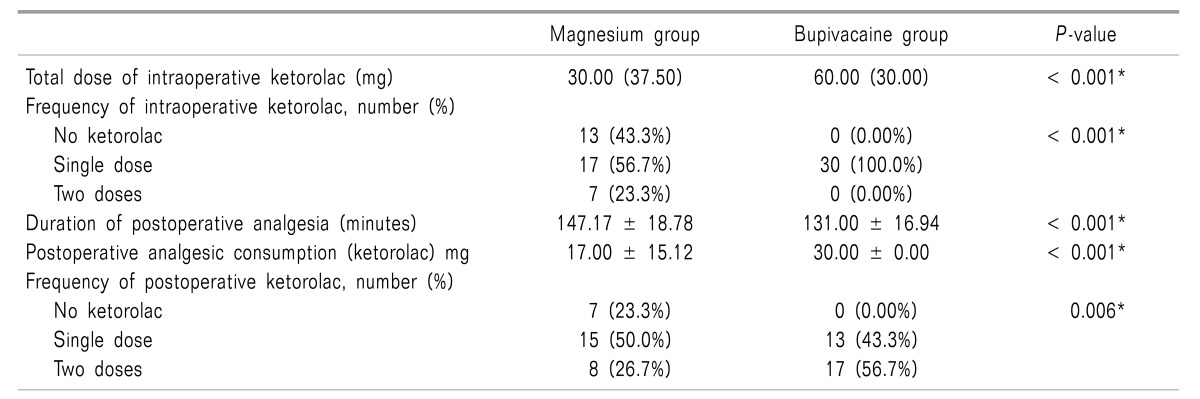
Intraoperative analgesia consumption (ketorolac) was significantly lower in the Magnesium group than in the Bupivacaine group, (31.00 ± 21.55 mg and 47.00 ± 15.12 mg in both groups respectively), (P > 0.05) (Table 2 and Fig. 2).
Postoperative pain assessment using the VAS was statistically significantly lower in the Magnesium group after 2 hours compared with that of the Bupivacaine group, (P <0.001), but after that, small differences between both groups were found (Fig. 3).
Duration of postoperative analgesia was significant longer in the Magnesium group than in the Bupivacaine group, (147.17 ± 18.78 min. and 131.00 ± 16.94 min. respectively) (P < 0.001). Postoperative analgesic consumption (ketorolac) was significantly lower in the Magnesium group (17.00 ± 15.12 mg) than in the Bupivacaine group (30.00 ± 0.00 mg) (P < 0.001) (Table 2).
The onset of sensory block was significant later in the Magnesium group (4.00 ± 0.74 min) than in the Bupivacaine group (3.23 ± 1.25 min), with P = 0.005. The start of motor block was significant longer in the Magnesium group than in the Bupivacaine group (6.13 ± 0.86 min and 5.17 ± 1.49 min in both groups, respectively) with P = 0.003. Time to reach maximal sensory block was longer in the Magnesium group (6.60 ± 1.33 min) but not significantly longer than that in the Bupivacaine group (6.60 ± 1.33 min) (Table 3).
Postoperative nausea and vomiting in both groups showed an insignificant statistical difference between them (Table 4). Because the mean surgery time was around 115 min, the recording of the hemodynamic variable at the 150 min was recorded in the postanesthetic recovery unit. There were no statistically significant differences between the two groups as regarding the intraoperative and postoperative hemodynamic parameters (heart rate, blood pressure, and oxygen saturation).
Go to : 
The main finding of this study was that in patients undergoing gynecological laparoscopic surgery under hyperbaric Bupivacaine spinal anesthesia, the addition of (50 mg) Magnesium sulfate led to fewer analgesic requirements and less discomfort during the intraoperative and postoperative periods. Surgeon satisfaction was significant better in the Magnesium group than in the Bupivacaine group. There was a significant delay in the onset of sensory and motor blockade. To our knowledge, no published studies have investigated the impact of intrathecal Magnesium on surgeon satisfaction after gynecological laparoscopic surgery. The period of postoperative pain relief was significant longer in the Magnesium group than in the Bupivacaine group.
Many studies have compared surgical space conditions during laparoscopic cholecystectomy using low pressure with either profound or moderate neuromuscular blockade, and found that a deep block was better than a medium block in improving operational space condition and surgeon satisfaction [8].
Several studies demonstrated the analgesic effects of Magnesium when given with regional anesthesia [91011121314]. Ozalevli et al. [10], in 2005, observed that when Magnesium was added to Bupivacaine and an opioid (fentanyl) there was a prolongation of the period of anesthesia without additional side-effects. In some studies, it was observed that the pain scores, the first dose of analgesia required, and the total opioid requirement were significantly less after injection of Magnesium intrathecally [1112]. Unlugenc et al. [14] Showed that the addition of Magnesium to Bupivacaine did not affect the start of a sensory block or the period of spinal anesthesia. They said that these differences might be due to either the dose of intrathecal Bupivacaine and Magnesium, or different surgical techniques. Shariat Moharari et al. [15] in 2013 noticed that giving Magnesium intravenously led to less analgesic consumption and lower discomfort in the postoperative period. In the current study, the onset of the sensory blockade and the start of the motor blockade were significantly delayed with the addition of intrathecal Magnesium to Bupivacaine. This result is similar to previous research in which there was a delay in the start of both blockades with the addition of intrathecal Magnesium to Bupivacaine and fentanyl [101617].
However, it was in disagreement with other studies where no changes were found in the start of sensory and motor blockade or in the period of spinal anesthesia [16]. The differences in pH and baricity of the solution containing Magnesium contributed to the delayed onset [1017]. In disagreement with our study, Morrison et al. [18], in a systemic review and meta-analysis done in 2013, showed no effect from the addition of Magnesium to LA on the duration of spinal anesthesia, the start of sensory blockade, the motor blockade, or the incidence of side effects (hypotension, pruritus). However, with the addition of intrathecal opioids, there is a beneficial effect, which may suggest that Magnesium potentiates the effect of opioids.
Magnesium mediates its analgesic action by non-competitive inhibition of the NMDA receptor, blocking ion channels (calcium and sodium) in a voltage-dependent manner leading to central sensitization and wind-up attributed to peripheral nociceptive stimulation [19202122]. In chronic pain, calcium channel blockers potentiate the effects of morphine [23]. In previous studies, the ideal dose of intrathecal Magnesium has not been demonstrated. The dose of Magnesium in this research was guided by data from the earlier study of Buvanendran et al. [16] in 2002. We suggest further researchers in the future, with different Magnesium dosages and more patients, to determine the safest route and dosage.
This study demonstrates that intrathecal Magnesium sulfate as an adjuvant to Bupivacaine is desirable in patients undergoing gynecological laparoscopic surgery due to better surgeon and patient satisfaction with lower analgesic requirements intra-operatively and postoperatively.
Go to : 
ACKNOWLEDGEMENTS
Equipment used in this study was provided by Assiut University Hospitals, Assiut, Egypt. We received no additional funding.
The authors declare that they have no competing interests.
Go to : 
References
1. Sinha R, Gurwara AK, Gupta SC. Laparoscopic surgery using spinal anesthesia. JSLS. 2008; 12:133–138. PMID: 18435884.
2. Hamad MA, El-Khattary OA. Laparoscopic cholecystectomy under spinal anesthesia with nitrous oxide pneumoperitoneum: a feasibility study. Surg Endosc. 2003; 17:1426–1428. PMID: 12802665.

3. Gerges FJ, Kanazi GE, Jabbour-Khoury SI. Anesthesia for laparoscopy: a review. J Clin Anesth. 2006; 18:67–78. PMID: 16517337.

4. Murphy AA, Nager CW, Wujek JJ, Kettel LM, Torp VA, Chin HG. Operative laparoscopy versus laparotomy for the management of ectopic pregnancy: a prospective trial. Fertil Steril. 1992; 57:1180–1185. PMID: 1534771.

5. Minai H, Yamada K, Tashiro K, Yamamoto K. Anesthetic management for awake laparoscopic surgery for ectopic pregnancy in a patient with heterotopic pregnancy. Masui. 2005; 54:1313–1314. PMID: 16296379.
6. Lennox PH, Vaghadia H, Henderson C, Martin L, Mitchell GW. Small-dose selective spinal anesthesia for short-duration outpatient laparoscopy: recovery characteristics compared with desflurane anesthesia. Anesth Analg. 2002; 94:346–350. PMID: 11812696.

7. Pascual-Ramírez J, Gil-Trujillo S, Alcantarilla C. Intrathecal magnesium as analgesic adjuvant for spinal anesthesia: a meta-analysis of randomized trials. Minerva Anestesiol. 2013; 79:667–678. PMID: 23722295.
8. Staehr-Rye AK, Rasmussen LS, Rosenberg J, Juul P, Lindekaer AL, Riber C, et al. Surgical space conditions during low-pressure laparoscopic cholecystectomy with deep versus moderate neuromuscular blockade: a randomized clinical study. Anesth Analg. 2014; 119:1084–1092. PMID: 24977638.

9. Faiz SH, Rahimzadeh P, Sakhaei M, Imani F, Derakhshan P. Anesthetic effects of adding intrathecal neostigmine or magnesium sulphate to bupivacaine in patients under lower extremities surgeries. J Res Med Sci. 2012; 17:918–922. PMID: 23825989.
10. Ozalevli M, Cetin TO, Unlugenc H, Guler T, Isik G. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anaesthesia. Acta Anaesthesiol Scand. 2005; 49:1514–1519. PMID: 16223399.

11. Shoeibi G, Sadegi M, Firozian A, Tabassomi F. The additional effect of magnesium sulfate to lidocaine in spinal anesthesia for cesarean section. Int J Pharmacol. 2007; 3:425–427.

12. Arcioni R, Palmisani S, Tigano S, Santorsola C, Sauli V, Romanò S, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce post-operative analgesic requirements: a prospective, randomized, double-blind, controlled trial in patients undergoing major orthopedic surgery. Acta Anaesthesiol Scand. 2007; 51:482–489. PMID: 17378788.

13. Ghrab BE, Maatoug M, Kallel N, Khemakhem K, Chaari M, Kolsi K, et al. Does combination of intrathecal magnesium sulfate and morphine improve postcaesarean section analgesia? Ann Fr Anesth Reanim. 2009; 28:454–459. PMID: 19427159.
14. Unlugenc H, Ozalevli M, Gunduz M, Gunasti S, Urunsak IF, Guler T, et al. Comparison of intrathecal magnesium, fentanyl, or placebo combined with bupivacaine 0.5% for parturients undergoing elective cesarean delivery. Acta Anaesthesiol Scand. 2009; 53:346–353. PMID: 19173689.

15. Shariat Moharari R, Motalebi M, Najafi A, Zamani MM, Imani F, Etezadi F, et al. Magnesium can decrease postoperative physiological ileus and postoperative pain in major non laparoscopic gastrointestinal surgeries: a randomized controlled trial. Anesth Pain Med. 2013; 4:e12750. PMID: 24660146.

16. Buvanendran A, McCarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ. Intrathecal magnesium prolongs fentanyl analgesia: a prospective, randomized, controlled trial. Anesth Analg. 2002; 95:661–666. PMID: 12198056.

17. Banihashem N, Hasannasab B, Esmaeili A, Hasannasab B. Addition of intrathecal magnesium sulfate to bupivacaine for spinal anesthesia in cesarean section. Anesth Pain Med. 2015; 5:e22798. PMID: 26161320.

18. Morrison AP, Hunter JM, Halpern SH, Banerjee A. Effect of intrathecal magnesium in the presence or absence of local anaesthetic with and without lipophilic opioids: a systematic review and meta-analysis. Br J Anaesth. 2013; 110:702–712. PMID: 23533255.

19. Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991; 44:293–299. PMID: 1828878.

20. Ascher P, Nowak L. Electrophysiological studies of NMDA receptors. Trends Neurosci. 1987; 10:284–288.

21. Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993; 77:362–379. PMID: 8346839.

22. Liu HT, Hollmann MW, Liu WH, Hoenemann CW, Durieux ME. Modulation of NMDA receptor function by ketamine and magnesium: part I. Anesth Analg. 2001; 92:1173–1181. PMID: 11323343.

23. Santillán R, Maestre JM, Hurlé MA, Flórez J. Enhancement of opiate analgesia by nimodipine in cancer patients chronically treated with morphine: a preliminary report. Pain. 1994; 58:129–132. PMID: 7970835.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download