Abstract
Background
Current therapy for the treatment of neuropathic pain is often unsatisfactory. Considerable variation in treatment pattern still exists in spite of availability of sufficient literature from various guidelines. Recent Indian market data suggested that the utilization (sale) of drugs such as amitriptyline, pregabalin, and gabapentin was more for low-dose unit packs than that of the high-dose unit packs, raising the belief that these drugs are prescribed at a lower dose than is actually recommended in the guidelines. To test this hypothesis, a survey was conducted across speciality throughout the country to observe the prescription pattern of these drugs amongst the health care providers in India.
Methods
Three hundred fifty survey forms were distributed of which 281 forms were included for analysis.
Results
It was observed that the commonly used initiation and maintenance dose for amitriptyline, pregabalin, and gabapentin was 5–10 mg/day, 50–75 mg/day, and 100–300 mg/day, respectively. The reason to select the lower dosages was to have a balancing effect to achieve good efficacy with minimum side effects. Care-givers reported no side effects/not many side effects as a reason in 22.2%, 16.88%, and 23.86% patients with amitriptyline, pregabalin, and gabapentin, respectively. Sedation and giddiness were commonly reported with all three drugs.
Go to : 
Treatment of neuropathic pain is challenging due to its varied etiology, symptoms and underlying mechanisms [12]. Uncertainty regarding the nature and exact location of the lesion, especially in non-specialist settings adds to this challenge. Various pharmacological options are available to manage neuropathic pain outside of speciality pain clinics. Treatment guidelines from various pain societies and working groups provide sufficient literature to guide the care-givers in optimal usage of the available drugs in the management of neuropathic pain [345].
In spite of this, there still exists a considerable variation in the way care givers initiate the treatment, maintain it, and the dosages they use for various medicines [6]. Variation is also observed amongst caregivers regarding the order in which the drugs are introduced in the therapy for managing the neuropathic pain. Literature also reports instances of non-adherence to the treatment guidelines and recommended doses used for the management of pain [7].
Tricyclic antidepressants and gabapentinoids constitute the first line drugs recommended by various guidelines for the management of neuropathic pain [38]. Indian market survey data (sales data) suggested that low dose unit packs of amitriptyline, pregabalin, and gabapentin were preferred to (had higher sales than) the high dose unit packs [9]. This suggests that these drugs may be prescribed at a lower dose than what is actually recommended. To test this hypothesis, we evaluated the prescription pattern of these first line drugs (amitriptyline, pregabalin, and gabapentin) for the management of neuropathic pain amongst caregivers. Other classes of first-line drugs used for the management of neuropathic pain are not included in this survey.
Go to : 
Indian market survey data (IMS) as of August 2016 suggested that the utilization (sale) of amitriptyline, pregabalin, and gabapentin was more in low-dose unit packs than in high-dose unit packs. There are lots of published studies on the etiology, pathophysiology, and pharmacological management of neuropathic pain. However, relatively little has been reported about the prescription pattern of these drugs used in the management of neuropathic pain in India.
This served as the basis to conduct a survey across India to observe the prescription pattern of the drugs used by the health care providers for the management of neuropathic pain. This data would guide primary care physicians to provide a balanced management approach based on both the efficacy and safety of the drug. Testing this hypothesis would be helpful to caregivers for strengthening their confidence in drug usage. It would further improve patients' compliance and adherence to treatment if low doses were found to be efficacious.
This was a questionnaire based feedback survey conducted all across India in thirty-one cities (East = 8, West = 8, North = 7, and South = 8 cities). Feedback questionnaire were equally distributed amongst 350 doctors in different zones across India, and 281 doctors completed the survey. The survey was spread across all doctor specialties having significant neuropathic pain practice. Incomplete feedback questionnaires were excluded from the survey. A majority of those involved were orthopedicians (95 doctors), physicians practicing internal medicine (91 doctors), and general practitioners (78 doctors). Rest of the group included doctors from a few other specialities (17 doctors).
Written responses were noted in the case record form (CRF). The CRF included the details of the healthcare provider with his speciality and place of practice. None of the answers were validated by looking at the actual prescription. Drugs surveyed in the study include amitriptyline, pregabalin, and gabapentin. Questions assessing details of the dosage used for initiation and maintenance therapy, reasons for selecting the particular dose, and the number of occurrences and percentage of side effects for the above mentioned drugs were recorded in the survey.
Observations were collected and entered in Microsoft Excel 2007 for analysis. Charts and graphs were derived from the collected data.
Go to : 
A total of 281 doctors took part in the survey conducted across India. An overview of the specialities is included in (Table 1).
Of the total 281 doctors, 244 (86.83%) used amitriptyline, 249 (88.61%) used pregabalin, and 164 (58.36%) used gabapentin in their clinical practice for the treatment of neuropathic pain. Responses of the doctors prescribing the three drugs are captured in terms of both initiation and maintenance.
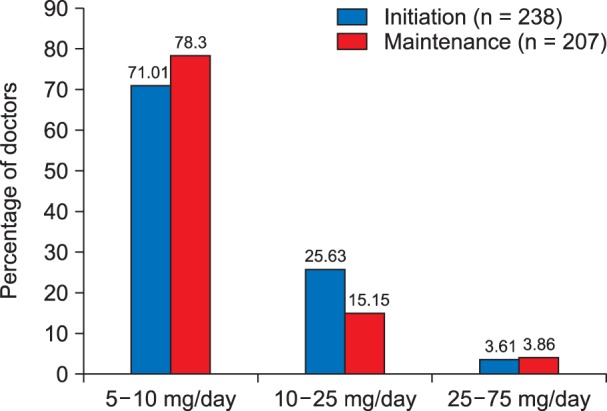
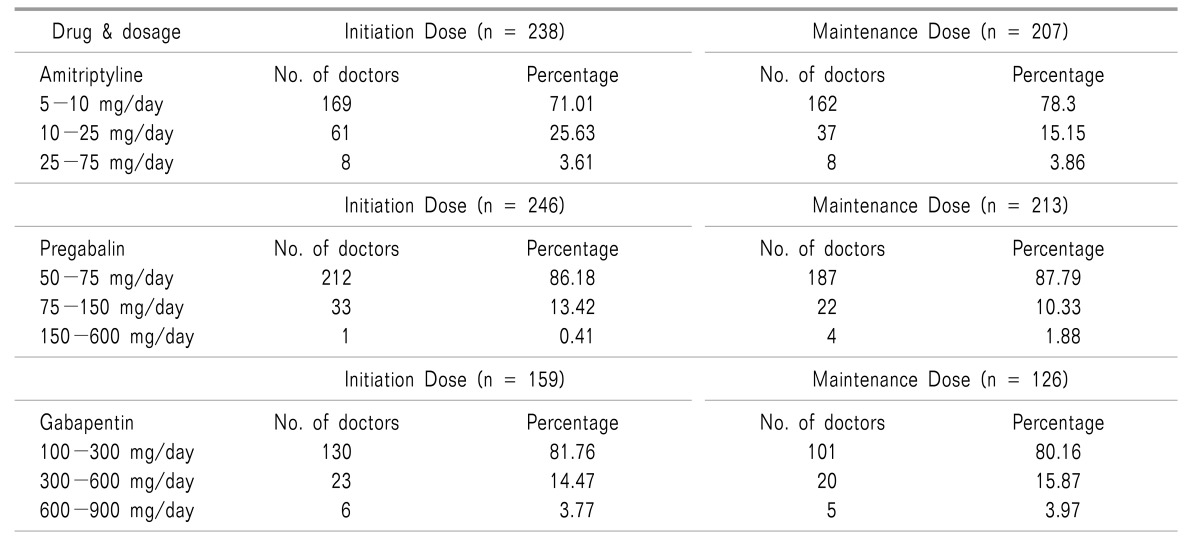
It was observed that 71.01% of the caregivers initiated amitriptyline in the dose ranging from 5-10 mg per day, 25.63% of the caregivers used amitriptyline in the dose of 10-25 mg per day, while rest of them 3.61% initiated amitriptyline at a dose ranging from 25 mg up to 75 mg (Fig. 1). Similarly, it was observed that 78.3% of the caregivers continued using amitriptyline at a daily maintenance dose of 5-10 mg/day, 15.15% of the caregivers used amitriptyline in a dose of 10-25 mg per day for maintenance, while rest of them 3.86% used amitriptyline for maintenance therapy at a dose ranging from 25 mg up to 75 mg/day (Fig. 1, Table 2).
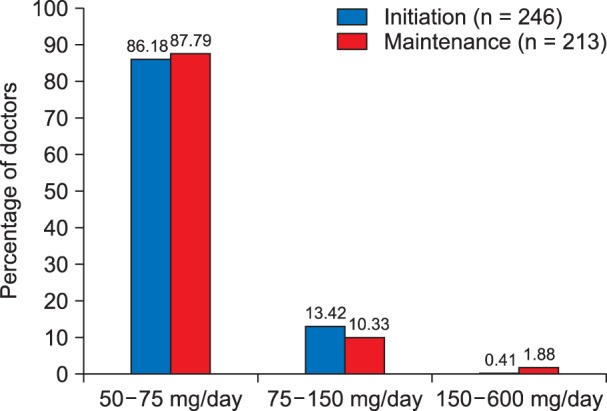
About 86.18% of the caregivers initiated pregabalin in a dose ranging from 50-75 mg per day, 13.42% of the caregivers initiated pregabalin in a dose ranging from 50-150 mg per day, while very few of them 0.41% initiated pregabalin at a dose higher than 150 mg/day up to 600 mg/day (Fig. 2). Similarly, it was observed that 87.79% of the caregivers continued using pregabalin at a daily maintenance dose of 50-75 mg/day, 10.33% of the caregivers used pregabalin in the dose of 75-150 mg per day for maintenance, while the rest of them (1.88%) used pregabalin for maintenance therapy at a dose of 150 mg to 600 mg/day (Fig. 2, Table 2).
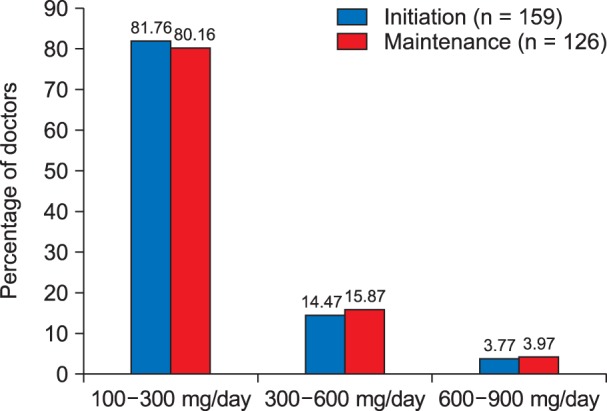
Most of the caregivers (81.76%) initiated gabapentin in a dose ranging from 100-300 mg per day, 14.47% of the caregivers initiated gabapentin in a dose ranging from 300-600mg per day, while very few of them (3.77%) initiated gabapentin at a dose ranging from 600-900 mg/day (Fig. 3). Similarly, it was observed that 80.16% of the caregivers continued using gabapentin at a daily maintenance dose of 100-300 mg/day, 15.87% of the caregivers used gabapentin in a dose of 300-600 mg per day for maintenance, while rest of them (3.97%) used gabapentin for maintenance therapy at a dose ranging from 600-900 mg/day (Fig. 3, Table 2).
A majority of caregivers (93.4%, 92.16%, and 90.03% for amitriptyline, pregabalin, and gabapentin, respectively) selected lower doses of amitriptyline (5-10 mg), pregabalin (50-75 mg/day), and gabapentin (100-300 mg/day) to balance the efficacy with safety. They felt that these doses resulted in better patient satisfaction and treatment compliance.
Few of them (3.7%, 6.09%, and 1.40% for amitriptyline, pregabalin, and gabapentin, respectively) were of the opinion that, these were the standard doses adequate for initiation and maintenance therapy. About 2.80%, 1.75%, and 8.57% of the prescribers for amitriptyline, pregabalin, and gabapentin respectively used the lower doses for titration based on the severity of symptoms.
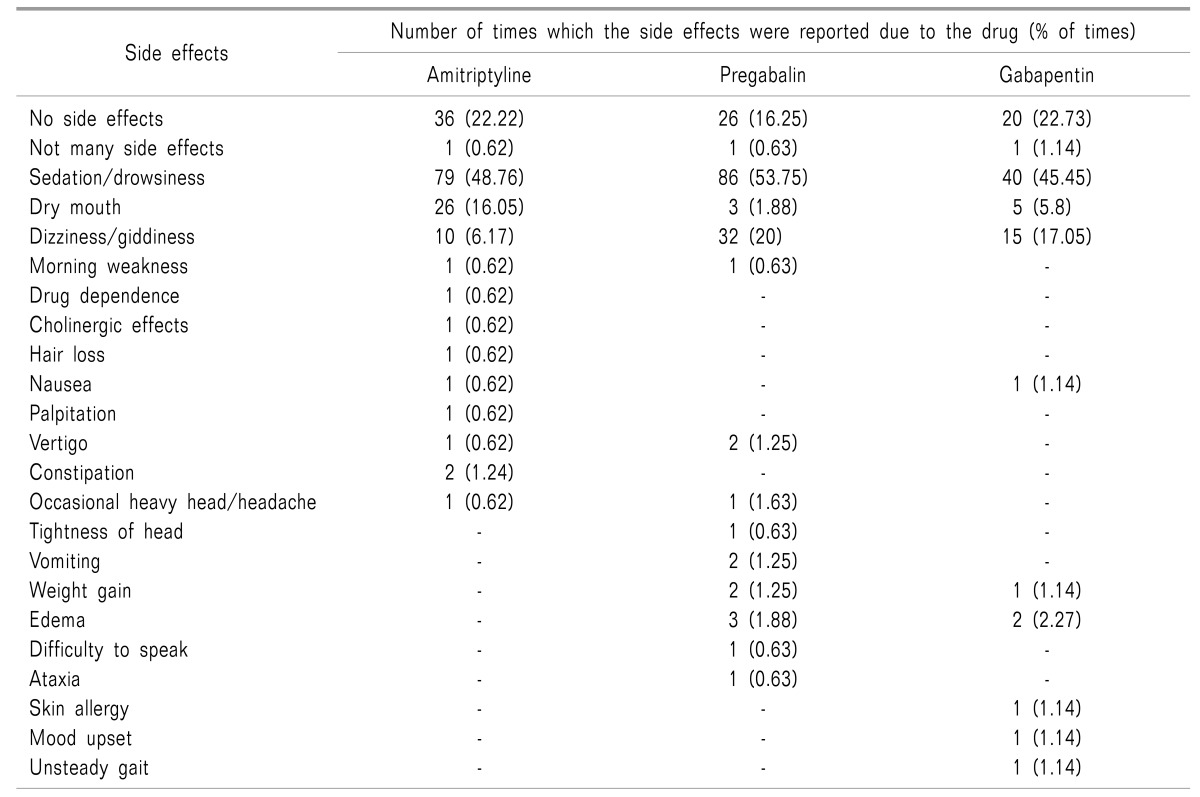
Sedation/drowsiness was reported 79 times (48.76%) as the associated side effect with the use of amitriptyline. This was followed by dry mouth, that was reported 26 times (16.05%). No side effects/not many side effects were reported 36 times (22.22%) in the survey in patients consuming amitriptyline (Table 3).
In patients advised pregabalin sedation/drowsiness/somnolence was reported 86 times (53.75%) as the associated side effect. This was followed by dizziness/giddiness that was reported 32 times (20%) in the survey by the health care provider. No side effects/not many side effects were reported 27 times (16.88%) in the survey (Table 3).
Similarly, sedation/drowsiness was reported 40 times (45.45%) as the associated side effect with the use of gabapentin. This was followed by dizziness/giddiness that was reported 15 times (17.05%) in the survey. No side effects/not many side effects were reported 21 times (23.86%) in the survey (Table 3).
Go to : 
The present study demonstrated that amitriptyline, pregabalin, and gabapentin were the commonly preferred drugs in the management of neuropathic pain in clinical practice. Of these 3 drugs amitriptyline and pregabalin were preferred by the caregivers to gabapentin. The non-linear pharmacokinetics of gabapentin and variable bioavailability requiring dose titration may be one of the reasons for its non-preference over pregabalin, however at the same time it was still favoured by a few, due to its time-tested efficacy even before the introduction of pregabalin, which still makes gabapentin a useful option [10].
Survey results revealed that amitriptyline, pregabalin, and gabapentin were prescribed in lower doses. These trends were in-line with the observed findings from the market data (sales data) which stated that the sale of low-dose unit packs was higher than that of high-dose unit packs. Interestingly these findings were similar across the three drugs (amitriptyline, pregabalin, and gabapentin), suggesting that Indian patients may perceive pain differently and respond to treatment in a different manner.
In the present survey, the patients responded well to the lower doses of amitriptyline, pregabalin, and gabapentin with minimum side effects. Lower doses were selected by the caregivers on purpose to balance the efficacy of the drug with the safety parameter. It was revealed that these (low) doses were well tolerated by the Indian patients resulting in better patient compliance. This holds true due to the given fact that occurrence of adverse events was one of the most common reasons for the interruption/stopping of treatment, observed in up to 40% of patients treated for pain related conditions [11]. In the published trials, the rate of treatment discontinuations observed among diabetic neuropathy patients ranged from 20% to 40% [12,13,14,15,16]. Similarly, the attrition rate observed for fibromyalgia ranged from 18% to 26% [17,18,19,20].
The most common prescribed dose (initiation and maintenance) of amitryptyline for the management of neuropathic pain ranged from 5-10 mg/day in the survey. Low dose administration of amitriptyline was also reported in the literature where it was initiated in a dose of 10 mg/day [21222324]. Suggested mechanisms for the analgesic action of low dose amitriptyline is suppression of response of peripheral C-type axons to nicotine by directly inhibiting nAChRs [25]. This inhibitory action on nAChRs in unmyelinated nociceptive axons may be an important component of amitriptyline's therapeutic effect in the treatment
of neuropathic pain.
Pregabalin was found to be commonly prescribed in a dose ranging from 25-75 mg/day in the survey. Similar findings were reported in the literature where pregabalin was prescribed in low doses in various conditions associated with pain [26,27,28,29,30,31]. One of these conditions included patients undergoing spinal surgery in which pregabalin was administered at a dose of 75 mg/day preoperatively and then 8 hourly thereafter [27]. Low-dose pregabalin (50 mg every 8 hours) was administered in cancer patients as an adjuvant to opioids for painful bone metastases [28]. Zin et al. [29] initiated pregabalin in a dose of 75 mg/day in patients with postherpetic neuralgia and painful diabetic neuropathy. Randinitis et al. [32] suggested dose reductions to be considered for pregabalin in older patients since oral clearance of pregabalin is likely to decrease with increasing age and patients with creatinine clearance of 30–60 ml/min are at greater risk of discontinuation due to adverse effects than patients having normal creatinine clearance.
Pooled analysis by Semel et al. [33] however demonstrated that the incidence of anticonvulsants observed with pregabalin was related to its dose rather than the age and hence it was necessary to titrate pregabalin to its lowest effective dose to minimize the risk of anticonvulsants in older patients treated for neuropathic pain.
In the survey, gabapentin was commonly prescribed at a dose of 100-300 mg/day. Literature although reports very few studies on low dose gabapentin administration one such trial was reported by Khurana et al. [27] in which gabapentin was administered preoperatively at a dose of 300 mg/day in patients undergoing spinal surgery and then every 8 hours for 7 days. It was observed that preoperative gabapentin administration was associated with less pain intensity and improved functional outcomes 3 months after lumbar discectomy. There were two cases where gabapentin was administered at lower doses for the management of sciatica [34]. In the first patient, gabapentin was initiated orally at a dose of 300 mg once/day after observing limited pain relief with non-steroidal anti-inflammatory drugs, opioids, and muscle relaxant. In the second patient gabapentin was initiated as 100 mg at bedtime and then titrated up to 100 mg twice/day with 200 mg at bed time. Both patients experienced significant pain relief. Arai et al. [35] administered gabapentin at a low dose of 400 mg/day along with antidepressants for the management of cancer pain. It was observed that low-dose gabapentin-imipramine significantly decreased the total pain score and daily paroxysmal pain episodes.
Response to treatment for neuropathic pain varies from individual to individual; as a result caregivers use their clinical judgements to titrate the dose per the tolerability of the patient rather than giving the full doses as suggested in the guidelines for the country. Non-adherence to the treatment guidelines and recommended doses for the management of pain are also reported in the literature. In the study by Liu et al. [7] was observed that patients with fibromyalgia often did not receive 1 of the prescription medications recommended by ACR guidelines and those who did were commonly prescribed lower-than-recommended doses, potentially resulting in poor effectiveness and tolerability. Our survey however was not targeted to measure the effectiveness of the response to pain treatment with the reported prescription pattern. Further trials investigating this parameter may add more value to this finding.
Treatment of neuropathic pain remains a challenge for the treating physician. Only 30-50% of patients get pain relief with the commonly prescribed drugs. Further titrating the dose at higher concentration for better pain relief adds to unwanted adverse effects resulting in poor treatment compliance. Also the observed efficacy and safety may vary with different patient populations. Market survey data suggested that low-dose unit packs of amitriptyline, pregabalin, and gabapentin are more utilized compared to the standard or the high-dose unit packs. To support this hypothesis a prescription analysis was conducted with Indian health care providers working in the field of neuropathic pain.
Observations revealed that Indian patients could be well-managed with minimal side effects with low dosages of amitriptyline, pregabalin, and gabapentin as compared to the standard doses. Efficacy of the lower doses needs to be further validated in a well-controlled trial in a larger population.
Go to : 
References
1. Nascimento OJ, Pessoa BL, Orsini M, Ribeiro P, Davidovich E, Pupe C, et al. Neuropathic pain treatment: still a challenge. Neurol Int. 2016; 8:6322. PMID: 27441065.

3. Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010; 85:S3–S14.

4. Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian pain society. Pain Res Manag. 2014; 19:328–335. PMID: 25479151.

5. Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010; 17:1113–1e88. PMID: 20402746.

6. NICE. Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. Centre for clinical practice at NICE (UK). London: National Institute for Health and Clinical Excellence (UK);2010. p. 1–138.
7. Liu Y, Qian C, Yang M. Treatment patterns associated with ACR-recommended medications in the management of Fibromyalgia in the United States. J Manag Care Spec Pharm. 2016; 22:263–271. PMID: 27003556.

8. Park HJ, Moon DE. Pharmacologic management of chronic pain. Korean J Pain. 2010; 23:99–108. PMID: 20556211.

9. Quintiles IMS Sales Data (Total Sales Audit and Secondary Sales Audit): Indian Pharmaceutical Market; Amitripyline, Gabapentin & Pregabalin; Moving Annual Total (MAT). 2016. 8.
10. Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49:661–669. PMID: 20818832.

11. Nantz E, Liu-Seifert H, Skljarevski V. Predictors of premature discontinuation of treatment in multiple disease states. Patient Prefer Adherence. 2009; 3:31–43. PMID: 19936143.

12. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005; 116:109–118. PMID: 15927394.

13. Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005; 6:253–260. PMID: 15820913.

14. Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003; 25:81–104. PMID: 12637113.

15. Dogra S, Beydoun S, Mazzola J, Hopwood M, Wan Y. Oxcarbazepine in painful diabetic neuropathy: a randomized, placebo-controlled study. Eur J Pain. 2005; 9:543–554. PMID: 16139183.

16. Vinik AI, Tuchman M, Safirstein B, Corder C, Kirby L, Wilks K, et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. Pain. 2007; 128:169–179. PMID: 17161535.

17. Patkar AA, Masand PS, Krulewicz S, Mannelli P, Peindl K, Beebe KL, et al. A randomized, controlled, trial of controlled release paroxetine in fibromyalgia. Am J Med. 2007; 120:448–454. PMID: 17466657.

18. Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE Jr, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multi-center trial. Arthritis Rheum. 2007; 56:1336–1344. PMID: 17393438.

19. Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005; 52:1264–1273. PMID: 15818684.

20. Arnold LM, Crofford LJ, Martin SA, Young JP, Sharma U. The effect of anxiety and depression on improvements in pain in a randomized, controlled trial of pregabalin for treatment of fibromyalgia. Pain Med. 2007; 8:633–638. PMID: 18028041.

21. Agius AM, Jones NS, Muscat R. A randomized controlled trial comparing the efficacy of low-dose amitriptyline, amitriptyline with pindolol and surrogate placebo in the treatment of chronic tension-type facial pain. Rhinology. 2013; 51:143–153. PMID: 23671895.

22. Kautio AL, Haanpää M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008; 35:31–39. PMID: 17980550.

23. Bowsher D. The management of postherpetic neuralgia. Postgrad Med J. 1997; 73:623–629. PMID: 9497970.

24. Kulshreshtha P, Gupta R, Yadav RK, Bijlani RL, Deepak KK. Effect of low-dose amitriptyline on autonomic functions and peripheral blood flow in fibromyalgia: a pilot study. Pain Med. 2012; 13:131–136. PMID: 22142408.

25. Freysoldt A, Fleckenstein J, Lang PM, Irnich D, Grafe P, Carr RW. Low concentrations of amitriptyline inhibit nicotinic receptors in unmyelinated axons of human peripheral nerve. Br J Pharmacol. 2009; 158:797–805. PMID: 19694730.

26. George RB, McKeen DM, Andreou P, Habib AS. A randomized placebo-controlled trial of two doses of pregabalin for postoperative analgesia in patients undergoing abdominal hysterectomy. Can J Anaesth. 2014; 61:551–557. PMID: 24668315.

27. Khurana G, Jindal P, Sharma JP, Bansal KK. Postoperative pain and long-term functional outcome after administration of gabapentin and pregabalin in patients undergoing spinal surgery. Spine (Phila Pa 1976). 2014; 39:E363–E368. PMID: 24384657.

28. Nishihara M, Arai YC, Yamamoto Y, Nishida K, Arakawa M, Ushida T, et al. Combinations of low-dose antidepressants and low-dose pregabalin as useful adjuvants to opioids for intractable, painful bone metastases. Pain Physician. 2013; 16:E547–E552. PMID: 24077205.
29. Zin CS, Nissen LM, O’Callaghan JP, Duffull SB, Smith MT, Moore BJ. A randomized, controlled trial of oxycodone versus placebo in patients with postherpetic neuralgia and painful diabetic neuropathy treated with pregabalin. J Pain. 2010; 11:462–471. PMID: 19962354.

30. Hounnou P, Nicoucar K. Delayed onset of rotatory self-motion perception, dysdiadochokinesia and disturbed eye pursuit caused by low-dose pregabalin. BMJ Case Rep. 2014; 4. 11. [serial on the Internet]. Available at http://casereports.bmj.com/content/2014/bcr-2013-201282.long.

31. Peng PW, Li C, Farcas E, Haley A, Wong W, Bender J, et al. Use of low-dose pregabalin in patients undergoing laparoscopic cholecystectomy. Br J Anaesth. 2010; 105:155–161. PMID: 20581215.

32. Randinitis EJ, Posvar EL, Alvey CW, Sedman AJ, Cook JA, Bockbrader HN. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol. 2003; 43:277–283. PMID: 12638396.

33. Semel D, Murphy TK, Zlateva G, Cheung R, Emir B. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010; 11:85. PMID: 21054853.

34. Grice GR, Mertens MK. Gabapentin as a potential option for treatment of sciatica. Pharmacotherapy. 2008; 28:397–402. PMID: 18294119.

35. Arai YC, Matsubara T, Shimo K, Suetomi K, Nishihara M, Ushida T, et al. Low-dose gabapentin as useful adjuvant to opioids for neuropathic cancer pain when combined with low-dose imipramine. J Anesth. 2010; 24:407–410. PMID: 20217150.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download