Abstract
Background
Current evidence suggests that intravenous magnesium sulfate might be effective for reducing migraine pain. In a recent pilot study, we showed that intravenous caffeine citrate could reduce the severity of migraine headache. The objective of this study is to investigate the efficacy of intravenous caffeine citrate vs. magnesium sulfate for management of acute migraine headache.
Methods
We conducted a prospective quasi-experimental study from January until May 2016 in two educational medical centers of Shahid Beheshti University of Medical Sciences (Shoahadaye Tajrish Hospital and Imam Hossein Hospital), Tehran, Iran. The study included patients who were referred to the emergency department and met the migraine diagnosis criteria of the International Headache Society. Patients were allocated into 2 groups receiving either 60 mg intravenous caffeine or 2 g intravenous magnesium sulfate. The pain scores, based on the visual analog scale, were recorded on admission, as well as one and two hours after receiving the drug. A Chi-Square test and student t-test were used for analysis of baseline characteristics. A Mann-Whitney U test and Wilcoxon singed rank test were used to analyze differences in the visual analogue scale (VAS) score between and within the groups respectively.
Results
In total, 70 patients (35 patients in each group) with the mean age of 33.1 ± 11.3 years were included (64.3% female). For the Caffeine citrate group, the median pain score decreased from 9.0 (2.0) to 5.0 (4.0) after one hour and to 3.0 (4.0) after two hours. For the magnesium sulfate group, the pain score decreased from 8.0 (2.0) to 2.0 (2.0) after one hour and to 0.0 (1.0) after two hours. Both intravenous caffeine citrate and intravenous magnesium sulfate reduced pain scores significantly but the magnesium sulfate group showed more improvement than the Caffeine citrate group after one hour (P < 0.001) and after two hours (P < 0.001).
Conclusions
It is likely that both intravenous caffeine and intravenous magnesium sulfate can reduce the severity of migraine headache. Moreover, intravenous magnesium sulfate at a dose of 2 g might be superior to intravenous caffeine citrate 60 mg for the short term management of migraine headache in emergency departments.
Go to : 
Migraine is a prevalent, debilitating neurological condition that lacks a universally effective therapy. It is characterized by attacks of throbbing, unilateral pain associated with nausea, vomiting, phonophobia, and photophobia [1].
Migraine treatment in emergency departments presents a clinical appeal for both patients and health care providers. Patients' response to medications is widely idiosyncratic and treatment agents that were effective in one patient might fail in another apparently similar case. Therefore, the treatment must be tailored to each individual case. The mainstays of current migraine management options include non-steroidal anti-inflammatory drugs (NSAIDs) and triptans [2]. NSAIDs are used for mild to moderate attacks of migraine as well as acetaminophen, which is proven to be effective for attacks of moderate severity [34]. Unfortunately, NSAIDs have a short half-life; therefore, repeated administration may be needed on a single attack of migraine and adverse effects like tight throat and flushing are common [56]. Triptans are usually prescribed for severe attacks not responding to the NSAIDs/acetaminophen strategy [5]. However, the associated side effects limit their prescription in the clinic. The combination of analgesics with tramadol, barbiturates, or stronger opioids, like morphine, is restricted to exceptionally unresponsive cases. Thus safer, more tolerable, and mechanism-based treatment approaches for migraine are imperative.
Suboptimal magnesium level has been repeatedly reported in migraine patients [789]. Consequently, several clinical trials investigated the efficacy and safety of parenteral and oral magnesium supplementation in acute migraine [1011121314]. Some studies support the use of oral magnesium for the prophylaxis of migraine [15].
Caffeine has been recommended for acute migraine treatment for hundreds of years [16]. Combinations of caffeine with non-opioid analgesics, ergotamine and codeine showed an abortive effect in acute episodes of migraine [171819]. In two recent trials, we reported that intravenous caffeine has comparable efficacy to ketorolac for ameliorating migraine pain [2021].
There is scarce data on the efficacy of intravenous caffeine citrate for reducing pain scores in patients with acute migraine. We have done some studies about the role of caffeine for pain management in acute migraine headache [16]. We have assessed its efficacy as a sole treatment and also compared it with ketorolac [2021]. To the best of our knowledge, there is not any study that uses intravenous caffeine in this regard, except for those belonging to Baratloo et al. [2021]. To further these investigations, in the current study, we performed prospective research to assess the efficacy of intravenous caffeine citrate vs. magnesium sulfate for management of acute migraine headache.
Go to : 
This was a prospective quasi-experimental study that was conducted from January until May 2016 in two educational medical centers at Shahid Beheshti University of Medical Sciences (Shoahadaye Tajrish Hospital and Imam Hossein Hospital), Tehran, Iran. Patients with a chief complaint of moderate to severe headache presenting to the emergency department were considered for this study. For patient enrolment, we used the same strict inclusion and exclusion criteria reported in our previous pilot study [21].
The visual analog scale (VAS) is a measurement tool used to quantify the pain score. VAS is a continuous scale comprised of a horizontal or vertical line, usually 10 centimeters (100 mm) in length. In the current study we considered 10 as the highest possible VAS score. In this measurement scale, “no pain” was defined as a score of 0 and “pain as bad as it could be” or “the worst imaginable pain” was considered as a score of 10 [22].
Patients who referred to the emergency department were deemed eligible and included in the study if they met the following criteria: 1) their age ranged from 18 to 60 years old; 2) their complaint of migraine pain fulfilled the criteria of a common migraine based on the International Headache Society Criteria and they had had migraines for at least a year prior to the admission day [1]; and 3) their VAS pain score indicated severe or moderate pain (VAS pain score ≥ 4) [2324].
We excluded patients who had, at least, one of the following items: 1) a history of any cardiac dysrhythmia; 2) hypertension; 3) ischemic heart disease; 4) active peptic ulcer disease; 5) inflammatory bowel disease; 6) pregnancy; 7) were breast feeding; 8) coagulopathy; 9) renal failure; 10) hepatic failure; or 11) a sleep disorder.
The sample size was calculated to detect a change of at least 3 cm in the VAS scale; considering β = 0.01 and α = 0.05, the minimum sample size for the study was estimated to be 35 subjects in each group.
Those presenting to the emergency department of Shohadaye Tajrish Hospital received a 60 mg caffeine citrate intravenous infusion within about 10 minutes. Those referring to the Imam Hossein Hospital received an intravenous infusion of 2 g magnesium sulfate. Both drugs were diluted in 100 cc normal saline 0.9% and infused over 10 minutes. All patients were monitored for any possible side effects. If any adverse effects (tachycardia, hypertension, itching, nausea and vomiting, pain at the injection site, irritability) happened, the process had to be stopped. At one and two hours after drug administration, patients were asked about their pain score on the VAS scale. A decrease of more than 3 points on the pain scale was considered a proper response [25].
Statistical analysis was performed using SPSS version 22. For describing continuous variables and mean, standard deviation, median, and inter-quartile range were used. The Chi-Square test and student t-test were used for analysis of baseline characteristics. The Mann-Whitney U test and Wilcoxon signed rank test were used to analyze differences in VAS pain scores. A P value ≤ 0.05 was considered significant.
The study was performed with a strict commitment to ethical considerations of the Declaration of Helsinki. All eligible patients were informed about the new drug, and all gave a signed informed consent. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. This study is registered in the Iranian Registry of Clinical Trials (www.irct.ir) under the code IRCT2016061315640N2.
Go to : 
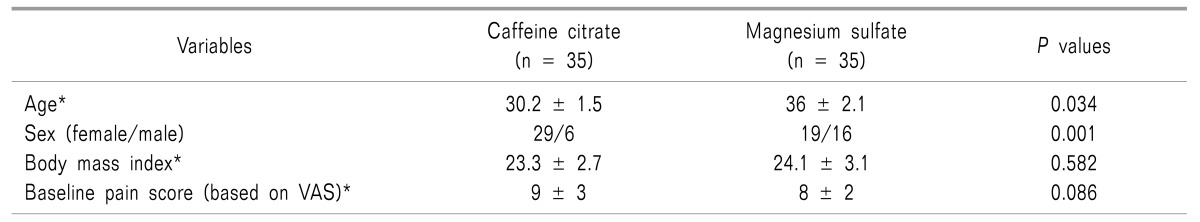
Seventy patients were enrolled in the study. Their mean age was 33.1 ± 11.3 years. Forty-five of the patients (64.3%) were female and 25 (35.7%) were male. Baseline characteristics of the patients in the two groups are shown in Table 1. Age and gender were significantly different in the two studied groups.
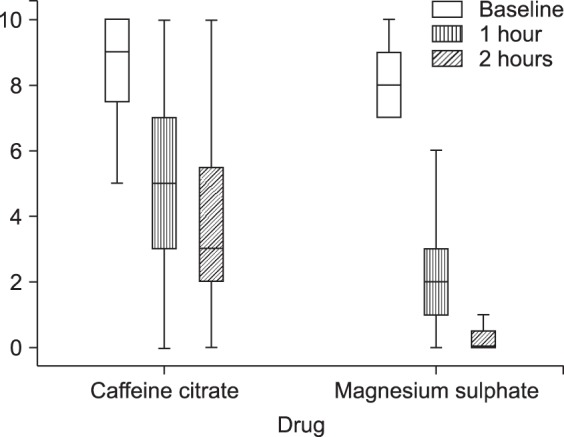
For the Caffeine citrate group, the median (interquartile range (IQR)) of the VAS pain score decreased significantly from 9.0 (2.0) to 5.0 (4.0) after one hour and to 3.0 (4.0) after two hours. For the Magnesium Sulfate group, the VAS pain score decreased significantly from 8.0 (2.0) to 2.0 (2.0) after one hour and to 0.0 (1.0) after two hours (Fig. 1).
The VAS pain score decreased more in the Magnesium sulfate group compared to the Caffeine citrate group after one hour (P < 0.001) and two hours (P < 0.001).
Go to : 
Headache is the most important and most common manifestation of migraine. Due to the pulsating nature of the headache, the pathophysiology of migraine was attributed to vascular problems in the brain for many years. The vascular theory for explaining migraine, which was considered valid until the middle of the 20th century, was based on 3 observations: 1) observations reported by 2 researchers named Ray and Wolff during a neurosurgical operation on a conscious patient in 1940; 2) inflation of carotid and extracranial arteries and seeing them pulsating during migraine headache attacks; 3) effectiveness of ergotamine, which is a powerful vasoconstrictor, for treating migraines [2627]. Currently, previous theories are questioned and more thorough investigations are being carried out on this topic. Modern imaging methods have shown that changes in vascular diameters are not proportionate with pain severity and response to treatment [28293031]. It seems that at the beginning of the headache vasodilation has not occurred yet, raising the question about the role of vasodilation in the pathophysiology of this disorder [32]. Therefore, migraine is not a primary vascular event and it seems that stimulation of trigeminal nerve branches as well as parasympathetic nerves following a series of activities in the brain's cortex (cortical spreading depression) leads to reactions in the meninges and brain stem that can explain migraine symptoms [33]. Disorders in the function of aminergic brain stem nuclei result in vasodilation and an increase in severity of pain through a series of reactions, and vasodilation brings about other neurogenic reactions. The close relationship of the vessels in the meninges with the trigeminal nerve branches helps in a better understanding of these events [34].
The results of this study showed that both intravenous magnesium sulfate and intravenous caffeine citrate could be considered for treatment of acute migraine headache. However, magnesium sulfate caused a more significant reduction of the VAS pain score compared to caffeine citrate at one and two hours after administration. However, there was a significant difference in age and sex between the studied groups that could be “confounding factors”.
There were no cases of withdrawal or intolerance to the treatment, which indicates the safety of intravenous caffeine citrate and magnesium sulfate in the studied population. There was not any serious adverse event in the studied population throughout the duration of hospitalization. However, this should be interpreted cautiously due to the short period of observation and the strict inclusion and exclusion criteria for selecting eligible subjects.
The molecular mechanism behind migraine has not yet been understood. However, the literature suggests multiple underlying mechanisms, which allows for testing a wide range of therapeutic options. Magnesium deficiency is hypothesized to be implicated in the pathophysiology of migraine. Physiologically, magnesium inactivates excitatory N-Methyl-D-aspartate (NMDA) glutamate receptors [35]. Depletion of magnesium drives NMDA-coupled calcium channels towards abnormal opening, which allows increased calcium influx, causing cytotoxicity and leading to neuronal injury secondary to the generation of toxic nitric oxide radicals [36]. Cortical depression is another possible mechanism for initiation of migraine [3738]. Magnesium deficiency affects mitochondrial oxidative phosphorylation and neuronal polarization, resulting in altered mitochondrial metabolism, which is suggested as increasing susceptibility to cortical depression [39]. On the other hand, the neurogenic theory suggests central sensitization or sensitization of the trigemino-vascular afferents of nociceptive neurons as a possible cause of migraine [404142]. Caffeine acts by inhibiting adenosine receptors (A1 and A2) in the brain, which explains its marked effect as an abortive agent in migraine attacks [16434445].
A recent meta-analysis of 21 randomized clinical trials (RCT) demonstrated the favorable effects of both intravenous and oral magnesium sulfate supplementation. Intravenous magnesium sulfate reduced acute migraine attacks within 15 to 45 minutes, 120 minutes, and 24 hours after initial infusion, while oral supplementation alleviated the frequency and intensity of the attacks [46].
Caffeine is frequently used in combination with other analgesics. The acetaminophen/aspirin /caffeine cocktail is classified as a level one option for acute migraine attacks by the American Headache Society, whilst the combination of caffeine and ergotamine is listed as level two [2]. We initiated the use of caffeine as monotherapy in a pilot study followed by an RCT comparing intravenous caffeine and ketorolac [2021]. Both regimens showed comparable positive effect in terms of pain relief, therapeutic success, and incidence of adverse events [2021].
Age and sex varied significantly between the groups in this research. The relationship of migraine pain with these characteristics is complicated and confusing. Bolay et al. [47] claimed that the impact of sex on migraine pain varied with age and significant changes were seen in women over 30 years old; but not in men. Sex also influences headache characteristics and migraine-associated symptoms, which vary across age groups, particularly in women. It has been reported that women have lower pain thresholds and tolerance for pain, but a superior capability to differentiate painful stimuli. In addition, sex differences in response to pain treatment have been proposed. Some have attributed such differences to sex hormones [484950]. Age related changes such as hypothalamic-pituitary-adrenal axis dysregulation and autonomic function changes could lead to increased pain sensitivity. On the other hand, some literature suggests that pain perception diminishes in old age. It seems that existing data regarding pain and aging are limited and equivocal and further studies are needed [5152]. Victor et al. [53] assessed age and sex specific patterns of migraine prevalence in 40892 individuals in the United States. Migraine prevalence was 2-fold higher in females (17.5% vs. 8.6%) but showed a dual peaking around the late 20s and 50 years of age in both sexes.
Our study has several strong points: patients were diagnosed according to the migraine diagnostic criteria of the International Headache Society and the severity of pain was assessed by the VAS score, which is a common and reliable measure of pain. There are some limitations: (1) the sample size is relatively small and (2) the study did not investigate the long-term efficacy of the drugs. Lack of randomization is the most important disadvantages of quasi-randomized studies [54]. There are statistical differences regarding age and sex between the studied groups that might have caused a bias in this investigation.
In light of the current evidence, we argue that there is no universal agreement on the treatment of acute migraine. Therefore, management of acute episodes should be individualized on a case to case basis due to the highly idiosyncratic effects of available agents. We recommend further well designed RCTs to confirm the findings of this study.
It is likely that both intravenous caffeine and intravenous magnesium sulfate can reduce the severity of migraine pain. Moreover, intravenous magnesium sulfate at a dose of 2 g might be superior to intravenous caffeine citrate 60 mg for short term management of migraine headaches.
Go to : 
ACKNOWLEDGEMENTS
We would like to express our special thanks to the Emergency Department staff of Shohadaye Tajrish and Imam Hossein Hospitals, Tehran, Iran.
Go to : 
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808. PMID: 23771276.
2. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the american headache society evidence assessment of migraine pharmacotherapies. Headache. 2015; 55:3–20. PMID: 25600718.

3. Prior MJ, Codispoti JR, Fu M. A randomized, placebo-controlled trial of acetaminophen for treatment of migraine headache. Headache. 2010; 50:819–833. PMID: 20236342.

4. Lipton RB, Baggish JS, Stewart WF, Codispoti JR, Fu M. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000; 160:3486–3492. PMID: 11112243.

6. Suthisisang C, Poolsup N, Kittikulsuth W, Pudchakan P, Wiwatpanich P. Efficacy of low-dose ibuprofen in acute migraine treatment: systematic review and meta-analysis. Ann Pharmacother. 2007; 41:1782–1791. PMID: 17878396.

7. Boska MD, Welch KM, Barker PB, Nelson JA, Schultz L. Contrasts in cortical magnesium, phospholipid and energy metabolism between migraine syndromes. Neurology. 2002; 58:1227–1233. PMID: 11971091.

8. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002; 42:242–248. PMID: 12010379.

9. Trauninger A, Pfund Z, Koszegi T, Czopf J. Oral magnesium load test in patients with migraine. Headache. 2002; 42:114–119. PMID: 12005285.

10. Cete Y, Dora B, Ertan C, Ozdemir C, Oktay C. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs. metoclopramide in the management of acute migraine attacks in the emergency department. Cephalalgia. 2005; 25:199–204. PMID: 15689195.

11. Demirkaya S, Vural O, Dora B, Topçuoğlu MA. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache. 2001; 41:171–177. PMID: 11251702.

12. Bigal ME, Bordini CA, Tepper SJ, Speciali JG. Intravenous magnesium sulphate in the acute treatment of migraine without aura and migraine with aura. A randomized, double-blind, placebo-controlled study. Cephalalgia. 2002; 22:345–353. PMID: 12110110.

13. Corbo J, Esses D, Bijur PE, Iannaccone R, Gallagher EJ. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann Emerg Med. 2001; 38:621–627. PMID: 11719739.

14. Köseoglu E, Talaslioglu A, Gönül AS, Kula M. The effects of magnesium prophylaxis in migraine without aura. Magnes Res. 2008; 21:101–108. PMID: 18705538.
15. Teigen L, Boes CJ. An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine. Cephalalgia. 2015; 35:912–922. PMID: 25533715.

16. Baratloo A, Rouhipour A, Forouzanfar MM, Safari S, Amiri M, Negida A. The role of caffeine in pain management: a brief literature review. Anesth Pain Med. 2016; 6:e33193. PMID: 27642573.

17. Diener HC, Pfaffenrath V, Pageler L, Peil H, Aicher B. The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study. Cephalalgia. 2005; 25:776–787. PMID: 16162254.

18. Goldstein J, Silberstein SD, Saper JR, Ryan RE Jr, Lipton RB. Acetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled study. Headache. 2006; 46:444–453. PMID: 16618262.

19. Silberstein SD, Winner PK, Chmiel JJ. Migraine preventive medication reduces resource utilization. Headache. 2003; 43:171–178. PMID: 12603635.

20. Baratloo A, Bafarani SA, Forouzanfar MM, Hashemi B, Friedman BW, Abdalvand A. Intravenous caffeine versus intravenous ketorolac for the management of moderate to severe migraine headache. Bangladesh J Pharmacol. 2016; 11:428–432.

21. Baratloo A, Negida A, El-Ashal G, Behnaz N. Intravenous caffeine for the treatment of acute migraine: a pilot study. J Caffeine Res. 2015; 5:125–129.

22. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011; 63(Suppl 11):S240–S252. PMID: 22588748.
23. Alschuler KN, Jensen MP, Ehde DM. Defining mild, moderate, and severe pain in persons with multiple sclerosis. Pain Med. 2012; 13:1358–1365. PMID: 22925457.

24. Zelman DC, Dukes E, Brandenburg N, Bostrom A, Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005; 115:29–36. PMID: 15836967.

25. Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001; 18:205–207. PMID: 11354213.

26. Ray BS, Wolff HG. Experimental studies on headache: pain-sensitive structures of the head and their significance in headache. Arch Surg. 1940; 41:813–856.
27. Silberstein SD, Lipton RB, Dalessio DJ. Wolff's headache and other head pain. 7th ed. New York (NY): Oxford University Press;2001. p. 606.
28. Lance JW, Goadsby PJ. Chapter 8: migraine: pathophysiology. In : Lance JW, Goadsby PJ, editors. Mechanism and management of headache. 7th ed. Oxford: Elsevier Butterworth-Heinemann;2005. p. 87–121.
29. Silberstein SD, Lipton RB, Goadsby PJ. Headache in clinical practice. Oxford: Isis Medical Media;1998. p. 250.
30. Ashina S, Bendtsen L, Ashina M. Pathophysiology of migraine and tension-type headache. Tech Reg Anesth Pain Manag. 2012; 16:14–18.

31. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013; 75:365–391. PMID: 23190076.

32. Olesen J, Friberg L, Olsen TS, Iversen HK, Lassen NA, Andersen AR, et al. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann Neurol. 1990; 28:791–798. PMID: 2285266.

33. Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002; 8:136–142. PMID: 11821897.

34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002; 346:257–270. PMID: 11807151.
35. Tepper SJ. Complementary and alternative treatments for childhood headaches. Curr Pain Headache Rep. 2008; 12:379–383. PMID: 18765145.

36. Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011; 51:484–501. PMID: 21352223.

37. Bhaskar S, Saeidi K, Borhani P, Amiri H. Recent progress in migraine pathophysiology: role of cortical spreading depression and magnetic resonance imaging. Eur J Neurosci. 2013; 38:3540–3551. PMID: 24118449.

38. Eikermann-Haerter K, Ayata C. Cortical spreading depression and migraine. Curr Neurol Neurosci Rep. 2010; 10:167–173. PMID: 20425031.

39. D'Andrea G, Leon A. Pathogenesis of migraine: from neurotransmitters to neuromodulators and beyond. Neurol Sci. 2010; 31(Suppl 1):S1–S7. PMID: 20464574.
40. Levy D. Migraine pain and nociceptor activation--where do we stand? Headache. 2010; 50:909–916. PMID: 20546325.

41. Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009; 8:679–690. PMID: 19539239.

42. Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003; 4:386–398. PMID: 12728266.

43. Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001; 24:31–55. PMID: 11283304.

44. Scher AI, Lipton RB, Stewart W. Risk factors for chronic daily headache. Curr Pain Headache Rep. 2002; 6:486–491. PMID: 12413408.

45. Tavares C, Sakata RK. Caffeine in the treatment of pain. Rev Bras Anestesiol. 2012; 62:387–401. PMID: 22656684.

46. Chiu HY, Yeh TH, Huang YC, Chen PY. Effects of intravenous and oral magnesium on reducing migraine: a meta-analysis of randomized controlled trials. Pain Physician. 2016; 19:E97–E112. PMID: 26752497.
47. Bolay H, Ozge A, Saginc P, Orekici G, Uludüz D, Yalın O, et al. Gender influences headache characteristics with increasing age in migraine patients. Cephalalgia. 2015; 35:792–800. PMID: 25424708.

48. Vallerand AH, Polomano RC. The relationship of gender to pain. Pain Manag Nurs. 2000; 1:8–15. PMID: 11710147.

49. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009; 10:447–485. PMID: 19411059.

50. Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000; 4:24–30. PMID: 10998712.

51. Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med. 2012; 13(Suppl 2):S27–S36. PMID: 22497745.

52. El Tumi H, Johnson MI, Dantas PB, Maynard MJ, Tashani OA. Age-related changes in pain sensitivity in healthy humans: a systematic review with meta-analysis. Eur J Pain. 2017; 21:955–964. PMID: 28230292.

53. Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010; 30:1065–1072. PMID: 20713557.

54. Harris AD, McGregor JC, Perencevich EN, Furuno JP, Zhu J, Peterson DE, et al. The use and interpretation of quasi-experimental studies in medical informatics. J Am Med Inform Assoc. 2006; 13:16–23. PMID: 16221933.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download