Abstract
Loin pain haematuria syndrome (LPHS) is an uncommon clinical entity that has divided renal physicians, pain practitioners, and even psychiatrists since its initial description. A relative paucity of data exists regarding the condition, with best practice guidelines lacking amid the existing threads of anecdotal experiences and variable follow-up observations. The aim of this article was to review the cumulative published experience of pain relief strategies for LPHS.
Go to : 
Loin pain haematuria syndrome (LPHS) describes a clinical entity first identified by Little et al. [1] in three female patients. While the understanding of this uncommon but painful condition has increased somewhat over the years amid numerous speculations about its pathophysiology, the symptoms originally described in 1967 by Little and colleagues remain a common denominator in this diagnosis of exclusion. Episodic haematuria (gross or microscopic) is accompanied by recurrent or persistent severe loin pain affecting one or both sides [2].
With pain reported to be sudden and lasting anywhere from hours to months in the absence of any detectable origin on extensive investigation [345], it would be fair to say that this pain syndrome represents one of the most distressing and debilitating clinical syndromes in modern genitourinary pain practice.
Having exhausted medical therapies including conventional analgesic medications and angiotensin converting enzyme inhibitors [5], exasperated practitioners of the algology arts have sought to alleviate the plight of their patients by developing and substantiating several interventional pain relief strategies aimed at the relief of loin pain in this condition.
Go to : 
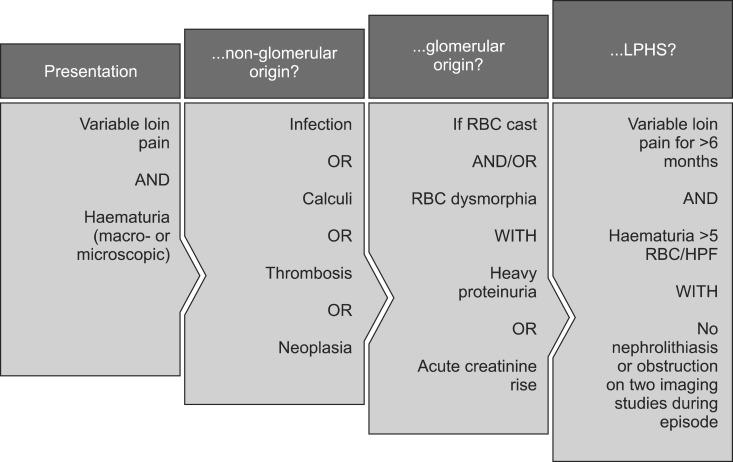
The lack of a consensus regarding tangible diagnostic criteria for LPHS in the age of modern medicine is testament to the scarce evidence base associated with the condition. Indeed, the "diagnosis of exclusion" status afforded to LPHS reflects the unfortunate but not uncommon reality of patients being seen by multiple physicians over several years before the diagnosis is finally stumbled upon. A sensible prelude to the diagnosis of LPHS, however, would appear to at least comprise the exclusion of non-glomerular and then glomerular causes of haematuria, before proceeding to a minimum six-month history of haematuria and loin pain not attributable to nephrolithiasis; a diagnostic guide is indicated in Fig. 1. The onset of symptoms usually occurs by the third decade of life and may be variable with respect to the pain component - sharp, intermittent pain has been described in contrast with a continuous ache [6]. Radiation from the loin to the abdomen or thigh, as well as classic radiation to the ipsilateral groin, may be absent or pronounced [7].
Epidemiological data related to this unusual pain syndrome indicate a prevalence of 0.012% [2], highlighting the rarity of this condition. The disease appears to display a preponderance for female patients (up to 70% in one series), with Caucasians at particular risk [8]. It may therefore serve clinicians well to consider LPHS as a diagnosis of exclusion when faced with as yet undiagnosed patients in this group.
As with diagnosticians before them, pathologists have faced difficulty in defining the characteristics of the syndrome by mutual consensus. Proposed pathophysiological concepts abound, with early theories focusing on renovascular anomalies followed by numerous suggestions in recent years. Associations between clinical LPHS findings and complement activation in the kidneys as well as microcrystal deposition have not been proven, although it is notable that up to half of LPHS patients do have a history of upper renal tract calculi [910]. A further suggestion involves thin glomerular basement membrane variants leading to filtrate backflow and consequent capsular stretch [11]. IgA nephritis has been described as responsible for an instance of "secondary LPHS" [1213], which in contrast to "primary LPHS" is attributable to an identified structural disease. Ultimately, however, many of the underlying mechanisms of the syndrome remain to be fully elucidated, leading clinicians to focus on supportive care via symptom management (pain control in particular) until we are in a better position to further understand this orphan disease.
Go to : 
Pain management of an uncommon condition often suffers the ignominy of being overlooked by major study groups, ostensibly due in part to difficulties with recruitment of sufficiently representative patient cohorts. Collation of published data pertaining to interventional pain management strategies in LPHS was therefore set as the aim of this review.
The PubMed database was searched on 18th May 2015 for items including the terms "loin pain" and "syndrome" in the title, and all matches were reviewed for mention of therapeutic strategies targeted at pain relief in LPHS. The search terms resulted in 90 potential sources of evidence, among which two lacked direct titular reference to LPHS and were excluded. The remainder were reviewed for direct titular mention of pain relief strategies, and 32 articles were identified. These were then directly evaluated, and significant or recurrent supported data were collated.
Go to : 
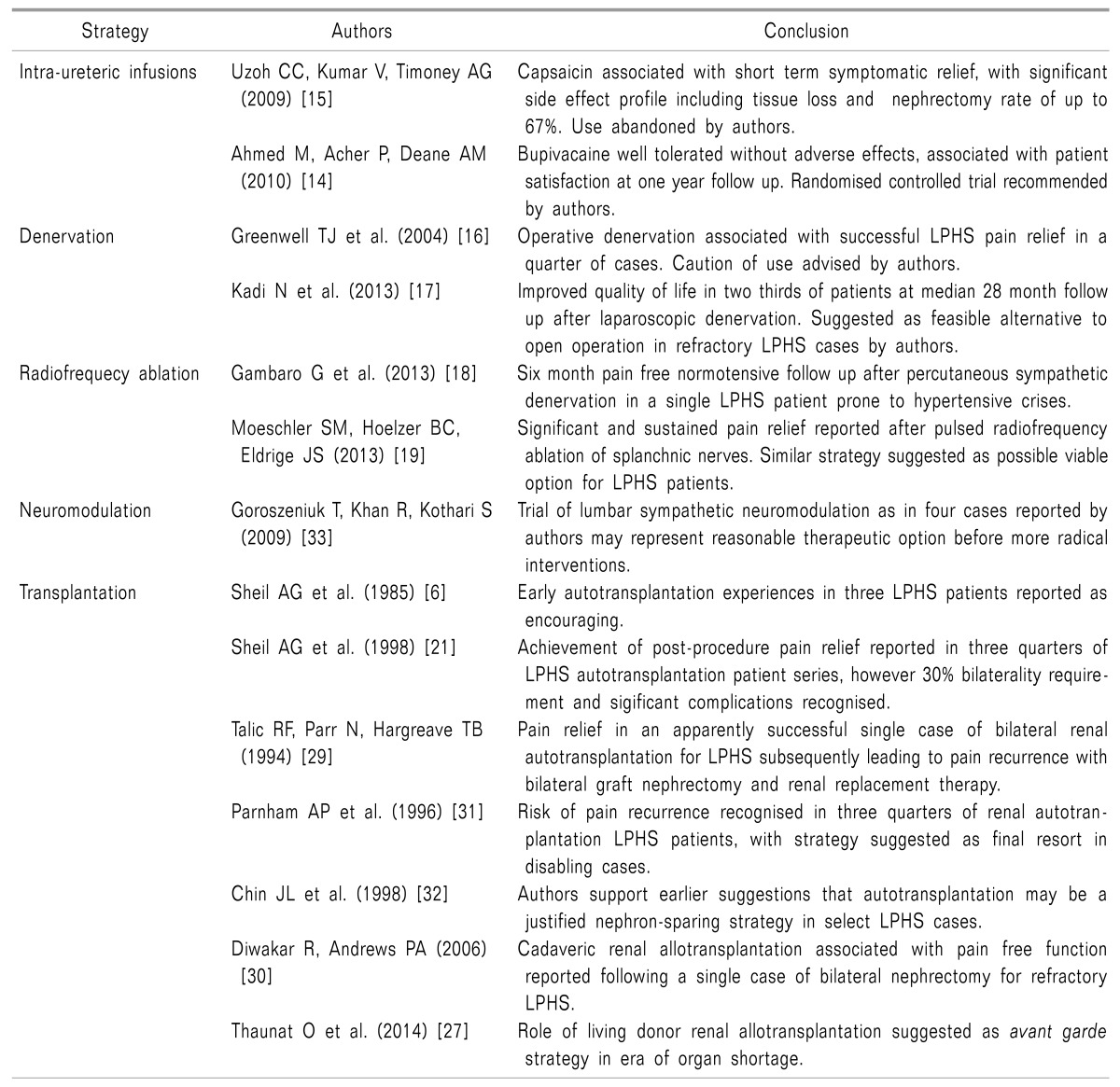
Intraureteric infusion of various substances via the flexible cystoscopy route with palliative and curative analgesic intent has been reported by numerous authors, albeit on lesser numbers of patients. In one of the more promising studies over the last few years, Ahmed et al. [14] demonstrated favourable potential for bupivacaine (0.5%) in the long-term treatment of loin pain. Endoscopic administration of 20 ml of 0.5% bupivacaine solution at a day procedure unit was reported in 17 patients with loin pain, among whom 12 had been diagnosed with LPHS. Follow-up at one year resulted in 16 patients expressing satisfaction with the improvement in their pain. One unhappy LPHS patient notwithstanding, four patients reported satisfaction after having received only one infusion, and the remaining 12 patients required repeat infusions at intervals ranging from three months to one year. While these data are encouraging, limitations of the authors' work include a suboptimal patient sample size for representative statistical extrapolations, the absence of a validated scoring system to quantify the scale of gains in pain control, and, rather significantly, the additional absence of a control population against which the results could feasibly be compared. An evidence lacuna therefore persists in the wake of this study in anticipation of the results of a randomised controlled trial. Regrettably, no major published data have as yet been made available in this regard since the work of Ahmed and colleagues [14] was brought to the international pain medicine stage in 2010.
Encouraging bupivacaine data aside, the intraureteric infusion of other pharmaceutical agents has been associated with a negative impression in the literature base. A systematic review by Uzoh et al. [15] addressed the intraureteric infusion of capsaicin with analgesic intent in LPHS patients, supplementing data reported over the five-year period from 2002 to 2007 with newly acquired data entries from the authors' own experiences. While conventional local anaesthetic-mediated ion channel interference is the presumed mechanism of action of intraureteric bupivacaine, the rationale for capsaicin administration is related to its potential for interaction with and reversible desensitisation of the type 1 vanilloid receptor (VR1). Yet following the analysis of data pertaining to a temporally and spatially convergent population of 52 patients with LPHS, capsaicin appeared to offer at best only short-term analgesia in a non-significant subset of cases. In addition to this disappointing data trend, a disconcerting side effect profile was noted, featuring not only urinary tract infection and bladder pain in treated patients, but also the worsening of LPHS symptoms in up to half of patients, and furthermore a concerning deterioration in renal function (not typically associated with the natural history of the LPHS state) in 20-50% of cases contributing to the overall pooled data. Were that not sufficient to disconcert any would-be capsaicin infusers, the authors go on to describe a 20-67% nephrectomy rate related to an increased nociceptive symptom burden and deterioration in the functional status of affected renal tracts.
Renal denervation has traditionally enjoyed time in the spotlight as a potential pain management strategy in LPHS cases refractory to non-interventional modalities, and may offer a less radical approach to the amelioration of painful symptoms and the attainment of improved quality of life in LPHS.
Retrospective analysis of data from 32 referred patients' experience of 41 renal surgical denervations under the care of the same operating surgeon was undertaken by Greenwell et al. [16] at the Institute of Nephrology and Urology, London, UK, in 2006. Of the 24 patients in whom full data sets were acquired, 33 denervations were reported, and these were associated with a mean follow-up period of 39.5 months.
Twenty-four out of the total 33 cases of renal denervation were identified as resulting in ipsilateral relapse of loin pain symptomatology, with this occurring at a median post-intervention interval of 11 months. Among the relapsing cases, nine patients proceeded to nephrectomy; within the relapse-nephrectomy group, three patients went on to manifest contralateral loin pain, while two patients reported debilitating wound pain. Among all of the denervation procedures, nine proved curative with regard to painful symptoms.
Now as then, author opinion advocates caution during consideration of renal denervation as an interventional pain relief strategy in LPHS patients; caring physicians should be mindful of the 25% curative denervation rate in this respect.
Minimally invasive denervation via laparoscopic surgical intervention has also been performed in recent years, with experiences of transperitoneal laparoscopic renal denervation undertaken in nine patients over ten years collated by Kadi et al. [17] in 2013. Nine female LPHS patients of a median age of 37 years underwent 11 laparoscopic denervation procedures between 2000 and 2010. Assessment of pain outcomes using the Pain Impact Questionnaire (PIQ-6TM) at a mean follow-up of 28 months revealed favourable data albeit of uncertain significance, with a curative intervention rate of 44% (median follow-up of 70.5 months). A reduction in analgesic requirement was reported in 22%, with 66.66% overall reporting a greater quality of life following laparoscopic intervention for LPHS.
Isolated reports have also suggested successful sympathetic renal nerve ablation via radiofrequency as an alternative to nephrectomy and auto-transplantation. Unilateral endoarterial renal sympathectomy was reported by Gambaro et al. [18] in conjunction with pain-free follow-up at six months, as well as drug-free normotension in the interval period despite patients having prior histories of recurrent hypertensive crises while on anti-hypertensive pharmacotherapy. The authors describe cannulation of the right renal artery via percutaneous femoral access with a Medtronic Symplicity™ Catheter (Medtronic, Dublin, Republic of Ireland) under radiographic and impedance control to target a zone of the arterial wall close to the renal hilum. Radiofrequency energy ablations of power 8W and duration two minutes were applied six times, along the length of the renal artery. Following unremarkable aortorenal angiography to assess post-procedure vessel integrity, patients experienced immediate favourable outcomes as above on the first day after intervention.
Contemporaneous data compiled by Moeschler et al. [19] appear to support pulsed radiofrequency as a potential therapeutic option in LPHS management, as in the instance of symptomatic relief of LPHS pain in a 50-year-old patient subjected to ablation of the splanchnic nerves after an initial favourable response to splanchnic nerve blockade.
Despite thus far presenting what again amounts to a lower level of evidence, authors continue to succeed in sowing the seeds for the development of mini-invasive interventional strategies in selected LPHS patient groups.
Early studies on pain management strategies in LPHS constituted little more than short case series with small numbers of patients. Three narcotic-dependent individuals with LPHS were described in a study by Sheil et al. [6], with unilateral symptomatology reported in two of these and predominant unilaterality in the final subject. With data from earlier studies involving splanchnic nerve blockade suggesting autonomic innervation as a mediator of nociceptive sensation [20], the authors were inspired to attempt renal excision and auto-transplantation as a means of pain relief in selected LPHS patients. Of the three patients, two reported remission from loin pain and the other episodic cramping in the region of the graft. A normal lifestyle was reported by all post-intervention subjects at a follow-up interval of 10-13 months. While recognising the fledging stage of their data as a limitation, the authors urged the pain community to be encouraged by the results.
Sheil went on to describe his cumulative experience of successive auto-transplantation with similar zeal some years later [21], in spite of an intervening account by another author of the recurrence of unilateral pain following sequential bilateral renal auto-transplantation for a diagnosis of LPHS in a single young female patient [22].
Symptomatic recurrence was further reported in a case report of a single patient debilitated by LPHS symptoms [23], and again in a further two patients who required narcotic analgesic therapy [24] - one for the recurrence of pain in the ipsilateral loin despite auto-transplantation, the other for chronic pain at the location of the transplanted kidney.
With gradual contributions to the literature base confirming several symptomatic relapses following auto-transplantation for LPHS, the psychological implications were broached in academia [25] after an analysis of a series of LPHS patients yielded suggestions of factitious disorder, major depressive disorder, post-traumatic stress disorder, personality disorders, and drug dependence. Subsequent studies reinforced the possibility of a significant somatoform element to the LPHS symptomatology [26]. A curious incident of perhaps particular interest to psychopathology and pain practitioners in this regard is the excellent outcome with respect to pain and nephrological function observed in two patients (followed up at 7 years and 8 months, respectively) recently described by Thaunat et al. [27] as recipients of nephrectomised LPHS kidneys.
The reported auto-transplantation case complicated by pain relapse and described by Hutchison et al. [22] in 1987 was subject to further pain community scrutiny by Gibson et al. [28] in 1994 when sequential bilateral nephrectomy and subsequent haemodialysis were embarked upon in the same patient in a final attempt at the alleviation of LPHS-associated pain. Curiously, the patient was reported to be experiencing a pain-free existence at the one-year follow-up, although regrettably no further follow-up data are available for this unique case of avant garde management.
A similar outcome of a bilateral auto-transplantation procedure followed by nephrectomies and ultimately renal replacement therapy for pain recurrence was reported later the same year by Talic et al. [29], with this representing (to the author's knowledge) the last documented case of radical renal tissue resection as attempted LPHS pain relief to be followed by long-term dialysis. A similar bilateral nephrectomy was indeed described by Diwakar and Andrews [30] in later years; however, the patient in the previously double auto-transplanted and subsequently nephrectomised and dialysed case study went on to receive a single cadaveric renal transplantation associated with pain-free follow-up at four years.
With increasing experience of auto-transplantation procedures accumulating with every passing year, authors began to propose risk factors and predictors of prognosis associated with this pain relief intervention of last resort; history of depressive disorder or absence of haematuria in the LPHS symptom spectrum were suggested as possible risk factors for poorer prognosis, while poor response to neurolytic block was described to predict failure of auto-transplantation [31]
The scattered case series and speculations put forward by a generation of specialist physicians in the field of LPHS were collated in a review by Chin et al. [32] in 1998. Follow-up data were analysed from the documented clinical courses of 22 patients who underwent a total of 26 reported renal auto-transplantations over the preceding twelve-year period, with success indicators including postoperative pain relief and narcotic use. With a follow-up period ranging from 30 to 138 months (mean follow-up period of 84.7 months), 18 auto-transplantation cases were associated with long-term relief after surgery. Six cases were associated with recurrence of pain (generally within the first twelve months after surgery); three of these underwent nephrectomy with subsequent symptomatic relief, two retained the transplanted kidney and made periodic use of narcotic analgesia, while the final case retained the transplanted kidney and reported non-narcotic analgesic use. Two of the 26 auto-transplantations were associated with early nephrectomy in view of ischaemia-related surgical complications.
A degree of heterogeneity in the published data notwithstanding, it would be fair to say, however, that a trend in outcomes has begun to establish itself in the literature.
In a novel attempt to demonstrate the potential role for an intermediate analgesic strategy prior to radical intervention in cases of intractable LPHS pain, Goroszeniuk et al. [33] proposed the concept of permanent electrode neuromodulation of the lumbar sympathetic chain. This procedure was undertaken in a series of four patients, initially via percutaneous monoelectrode insertion adjacent to the L3 and L4 vertebral bodies and subsequently via permanent implantation of a stimulatory system via four contact electrodes. Low-frequency stimulation resulted in successful achievement of an objective improvement in pain level utilising the visual analog scale, with significant amelioration in overall quality of life reported throughout the subject pool (including return to employment in one patient).
The premise of Gorszeniuk's intervention rests on the belief that visceral LPHS pain responds to neuromodulation of the autonomic supply to the involved kidney. While the results obtained by the authors do demonstrate promising findings for potential applications of neuromodulation in the supra-medical management of LPHS, limitations of the data interpretation include the small number of subjects in what essentially remains a non-contiguous series of selected reports.
Go to : 
Despite almost half a century having elapsed since the recognition of LPHS in the literature, the evidence-based management of severe and refractory LPHS pain is hindered by the scarcity of high-level evidence in favour of one strategy over another. A tendency toward radical intervention might be construed as an attempt at definitive management in selected, increasingly desperate LPHS patients in whom the condition significantly impacts quality of life. However, variable outcome data and lack of standardisation of long-term follow-up limit the extent to which one particular approach can be safely advocated. Evolving management strategies as listed in Table 1 show promise for future LPHS patients and pain practitioners alike, although until large multi-centre randomised controlled trials are seen, a sensible approach remains multi-disciplinary management shared between renal physicians, pain specialists, and psychologists/psychiatrists, as advocated by other authors.
Go to : 
References
1. Little PJ, Sloper JS, de Wardener HE. A syndrome of loin pain and haematuria associated with disease of peripheral renal arteries. Q J Med. 1967; 36:253–259. PMID: 4227314.
2. Taba Taba Vakili S, Alam T, Sollinger H. Loin pain hematuria syndrome. Am J Kidney Dis. 2014; 64:460–472. PMID: 24725981.

3. Leaker BR, Gordge MP, Patel A, Neild GH. Haemostatic changes in the loin pain and haematuria syndrome: secondary to renal vasospasm? Q J Med. 1990; 76:969–979. PMID: 2236480.
4. Lall R, Mailis A, Rapoport A. Hematuria-loin pain syndrome: its existence as a discrete clinicopathological entity cannot be supported. Clin J Pain. 1997; 13:171–177. PMID: 9186025.

5. Dube GK, Hamilton SE, Ratner LE, Nasr SH, Radhakrishnan J. Loin pain hematuria syndrome. Kidney Int. 2006; 70:2152–2155. PMID: 17051141.

6. Sheil AG, Ibels LS, Thomas MA, Graham JC. Renal autotransplantation for severe loin-pain/haematuria syndrome. Lancet. 1985; 326:1216–1217. PMID: 2866294.

7. Sherwood T. Loin pain/haematuria syndrome. Lancet. 1979; 313:1033–1034. PMID: 86752.
8. Zubair AS, Salameh H, Erickson SB, Prieto M. Loin pain hematuria syndrome. Clin Kidney J. 2016; 9:128–134. PMID: 26798473.

9. Coe FL, Evan AP, Worcester EM, Lingeman JE. Three pathways for human kidney stone formation. Urol Res. 2010; 38:147–160. PMID: 20411383.

10. Spetie DN, Nadasdy T, Nadasdy G, Agarwal G, Mauer M, Agarwal AK, et al. Proposed pathogenesis of idiopathic loin pain-hematuria syndrome. Am J Kidney Dis. 2006; 47:419–427. PMID: 16490620.

11. Hebert LA, Betts JA, Sedmak DD, Cosio FG, Bay WH, Carlton S. Loin pain-hematuria syndrome associated with thin glomerular basement membrane disease and hemorrhage into renal tubules. Kidney Int. 1996; 49:168–173. PMID: 8770964.

12. Smith HS, Bajwa ZH. Loin pain hematuria syndrome-visceral or neuropathic pain syndrome? Clin J Pain. 2012; 28:646–651. PMID: 22699133.

13. Jefferies ER, Phull JS, Gallegos CR. Loin pain haematuria syndrome. Ann R Coll Surg Engl. 2010; 92:360. PMID: 20501029.

14. Ahmed M, Acher P, Deane AM. Ureteric bupivicaine infusion for loin pain haematuria syndrome. Ann R Coll Surg Engl. 2010; 92:139–141. PMID: 20353642.

15. Uzoh CC, Kumar V, Timoney AG. The use of capsaicin in loin pain-haematuria syndrome. BJU Int. 2009; 103:236–239. PMID: 18727615.

16. Greenwell TJ, Peters JL, Neild GH, Shah PJ. The outcome of renal denervation for managing loin pain haematuria syndrome. BJU Int. 2004; 93:818–821. PMID: 15049996.

17. Kadi N, Mains E, Townell N, Nabi G. Transperitoneal laparoscopic renal denervation for the management of loin pain haematuria syndrome. Minim Invasive Ther Allied Technol. 2013; 22:346–351. PMID: 23688284.

18. Gambaro G, Fulignati P, Spinelli A, Rovella V, Di Daniele N. Percutaneous renal sympathetic nerve ablation for loin pain haematuria syndrome. Nephrol Dial Transplant. 2013; 28:2393–2395. PMID: 23658250.

19. Moeschler SM, Hoelzer BC, Eldrige JS. A patient with loin hematuria syndrome and chronic flank pain treated with pulsed radiofrequency of the splanchnic nerves. Clin J Pain. 2013; 29:e26–e29. PMID: 24104047.

20. Aber GM, Higgins PM. The natural history and management of the loin pain/haematuria syndrome. Br J Urol. 1982; 54:613–615. PMID: 6217860.

21. Sheil AG, Chui AK, Verran DJ, Boulas J, Ibels LS. Evaluation of the loin pain/hematuria syndrome treated by renal autotransplantation or radical renal neurectomy. Am J Kidney Dis. 1998; 32:215–220. PMID: 9708604.

22. Hutchison SM, Doig A, Jenkins AM. Recurrence of loin pain/haematuria syndrome after renal autotransplantation. Lancet. 1987; 329:1501–1502. PMID: 2885500.

23. Bloom PB, Viner ED, Mazala M, Jannetta PJ, Stieber AC, Simmons RL. Treatment of loin pain hematuria syndrome by renal autotransplantation. Am J Med. 1989; 87:228–232. PMID: 2667359.

24. Dimski DS, Hebert LA, Sedmak D, Ogrodowski JL, Elkhammas EA, Tesi RJ, et al. Renal autotransplantation in the loin pain-hematuria syndrome: a cautionary note. Am J Kidney Dis. 1992; 20:180–184. PMID: 1496973.

25. Kelly B. Psychological aspects of loin-pain/haematuria syndrome. Lancet. 1992; 340:1294. PMID: 1359353.
26. Lucas PA, Leaker BR, Murphy M, Neild GH. Loin pain and haematuria syndrome: a somatoform disorder. QJM. 1995; 88:703–709. PMID: 7493167.
27. Thaunat O, Kervella D, Matillon X, Rabeyrin M, Martin X, Morelon E, et al. Allotransplantation of kidney from unrelated living donor with loin pain haematuria syndrome. Transpl Int. 2014; 27:e24–e26. PMID: 24237133.

28. Gibson P, Winney RJ, Masterton G, Fowles RG. Bilateral nephrectomy and haemodialysis for the treatment of severe loin pain haematuria syndrome. Nephrol Dial Transplant. 1994; 9:1640–1641. PMID: 7870355.
29. Talic RF, Parr N, Hargreave TB. Anephric state after graft nephrectomy in a patient treated with renal autotransplantation for bilateral metachronous loin pain/hematuria syndrome. J Urol. 1994; 152:1194–1195. PMID: 8072094.

30. Diwakar R, Andrews PA. Renal transplantation in a patient with loin pain hematuria syndrome. Clin Nephrol. 2006; 66:144–146. PMID: 16939073.

31. Parnham AP, Low A, Finch P, Perlman D, Thomas MA. Recurrent graft pain following renal autotransplantation for loin pain haematuria syndrome. Br J Urol. 1996; 78:25–28. PMID: 8795395.

32. Chin JL, Kloth D, Pautler SE, Mulligan M. Renal autotransplantation for the loin pain-hematuria syndrome: long-term followup of 26 cases. J Urol. 1998; 160:1232–1235. PMID: 9751325.

33. Goroszeniuk T, Khan R, Kothari S. Lumbar sympathetic chain neuromodulation with implanted electrodes for long-term pain relief in loin pain haematuria syndrome. Neuromodulation. 2009; 12:284–291. PMID: 22151418.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download