Abstract
Background
Although opioids are the most commonly used medications to control postoperative pain in children, the analgesic effects could have a large inter-individual variability according to genotypes. The aim of this study was to investigate the association between single nucleotide polymorphisms and the analgesic effect of morphine for postoperative pain in children.
Methods
A prospective study was conducted in 88 healthy children undergoing tonsillectomy, who received morphine during the operation. The postoperative pain score, frequency of rescue analgesics, and side effects of morphine were assessed in the post-anesthesia care unit. The children were genotyped for OPRM1 A118G, ABCB1 C3435T, and COMT Val158Met.
Results
Children with at least one G allele for OPRM1 (AG/GG) had higher postoperative pain scores compared with those with the AA genotype at the time of discharge from the post-anesthesia care unit (P = 0.025). Other recovery profiles were not significantly different between the two groups. There was no significant relationship between genotypes and postoperative pain scores in analysis of ABCB1 and COMT polymorphisms.
Go to : 
Management of acute postoperative pain, especially in children, is a major concern of anesthesiologists. Although opioids are the most commonly used medications to control postoperative pain, the analgesic effects show large interindividual variability according to age, gender, ethnicity, type or duration of surgery, and so on [12]. Recently a number of studies have shown that single nucleotide polymorphisms (SNPs) in the pain pathway relate to variability in pain perception or response to analgesics following a painful procedure [345678].
A118G (rs1799971) in the mu-opioid receptor-1 gene (OPRM1) is one of the most well-known SNPs for modulating the analgesic response to morphine. The variation has been reported to reduce the analgesic effect of opioids as well as complications such as respiratory depression [345]. C3435T (rs1045642) in the adenosine triphosphate biding cassette gene (ABCB1) and Val158Met (rs4680) in the catechol-O-methyltransferase gene (COMT) are other SNPs of interest for postoperative pain management [678]. The aim of this study was to investigate the association between such SNPs and the analgesic effects of morphine for postoperative pain in children.
Go to : 
The protocol was approved by the Institutional Review Board of Jeju National University Hospital (2012-03-001). Written informed consent from parents and written assent from the children were obtained preoperatively. This study was conducted using a prospective observational method.
Korean children aged 5-18 years, American Society of Anesthesiologists (ASA) physical status 1 or 2, undergoing tonsillectomy with or without adenoidectomy, were enrolled in the study. Patients were excluded if they were affected by developmental delay, were diagnosed with hepatic or renal diseases, or experienced preoperative pain requiring analgesics (e.g., chronic tonsillitis).
General anesthesia was induced with propofol 2 mg/kg and morphine 0.05 mg/kg. Tracheal intubation was facilitated with rocuronium 0.6 mg/kg, and ondansetron 0.1 mg/kg was administered as a prophylactic antiemetic. Anesthesia was maintained with sevoflurane in 50% O2/N2O and the lungs were ventilated to maintain an end-tidal CO2 of 30-35 mmHg. Reversal agents for the muscle relaxant and morphine 0.05 mg/kg were injected just before the end of the operation. After the recovery of spontaneous respiration, patients were extubated and transferred to the post-anesthesia care unit (PACU).
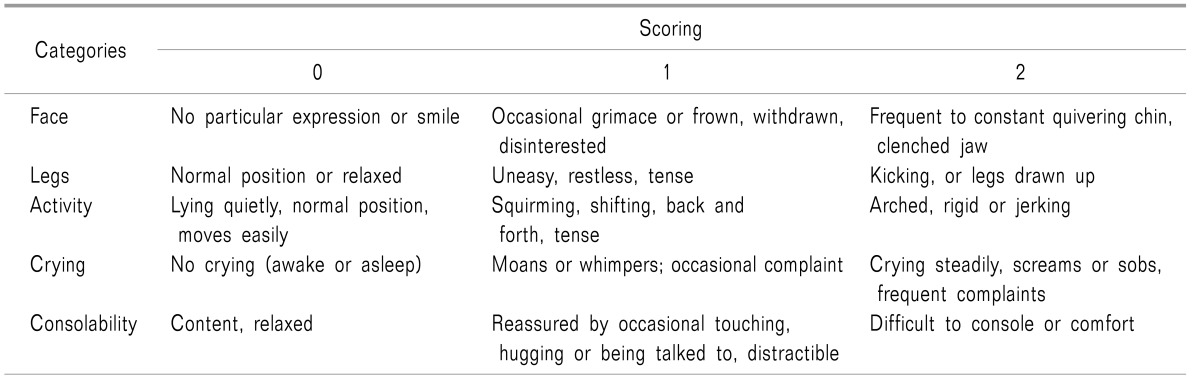
The primary outcome of this study was the postoperative pain score in the PACU. This was assessed using the Face, Legs, Activity, Crying, Consolability (FLACC) scale (Table 1) [9] upon arrival in the PACU, 15 min after PACU arrival, and upon discharge from the PACU. If pain persisted, ketorolac 0.01 mg/kg (for a pain score of 5-7) or morphine 0.05 mg/kg (for a pain score of ≥ 8) was administered for rescue analgesia.
Recovery profiles were assessed including the sedation score using the modified Ramsay sedation scale [10], respiratory depression, nausea/vomiting, and pruritus in the PACU. Patients stayed in the PACU for at least 30 min and were discharged after achieving a modified Aldrete score [11] of ≥ 9 and a pain score of ≤ 6. If the patient received rescue analgesics, we did not limit the pain score for PACU discharge.
Blood samples of 3 ml were collected after the induction of general anesthesia for genotyping. A118G, C3435T, and Val158Met were analyzed by TaqMan® genotyping assays (Life Technologies Corporation, Applied Biosystems, Carlsbad, CA, USA).
In a pilot study, the mean difference and standard deviation of postoperative pain scores between OPRM1 genotypes were 1.5 and 2.4, respectively. A sample size of 82 was expected at alpha error 0.05, power 0.8, and effect size 0.625. Considering the likely dropout rate, a total sample size of 100 was determined for the current study.
SPSS (IBM SPSS Statistics 20 for Windows, Chicago, IL, USA) was used for statistical analysis. Categorical data were analyzed by the Chi-square test. As the variables were not normally distributed, the relation between genotypes and postoperative measurements was analyzed using the Mann-Whitney test or the Kruskal-Wallis test. Each allelic frequency was examined by the Hardy-Weinberg equilibrium test [12]. A value of P < 0.05 was considered statistically significant.
Go to : 
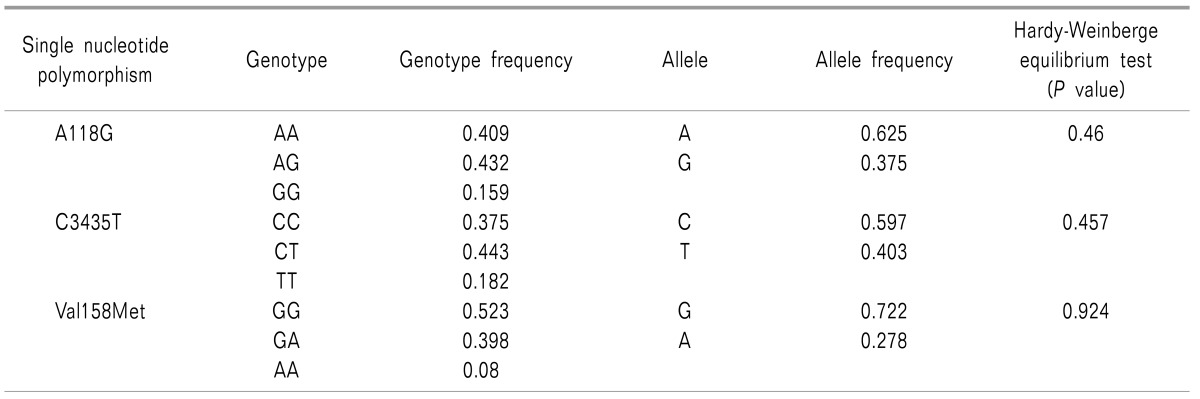
A total of 100 patients were enrolled in our study, of whom 12 patients were excluded due to problems with gene analysis or loss of other data. Thus, 88 patients were included in the final analysis. Genotypes and allelic frequencies of A118G, C3435T, and Val158Met are described in Table 2. All SNPs were in Hardy-Weinberg equilibrium.
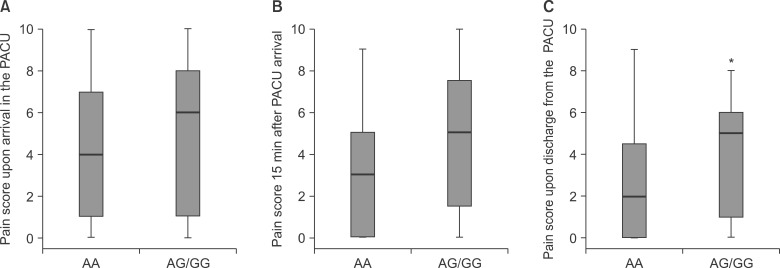
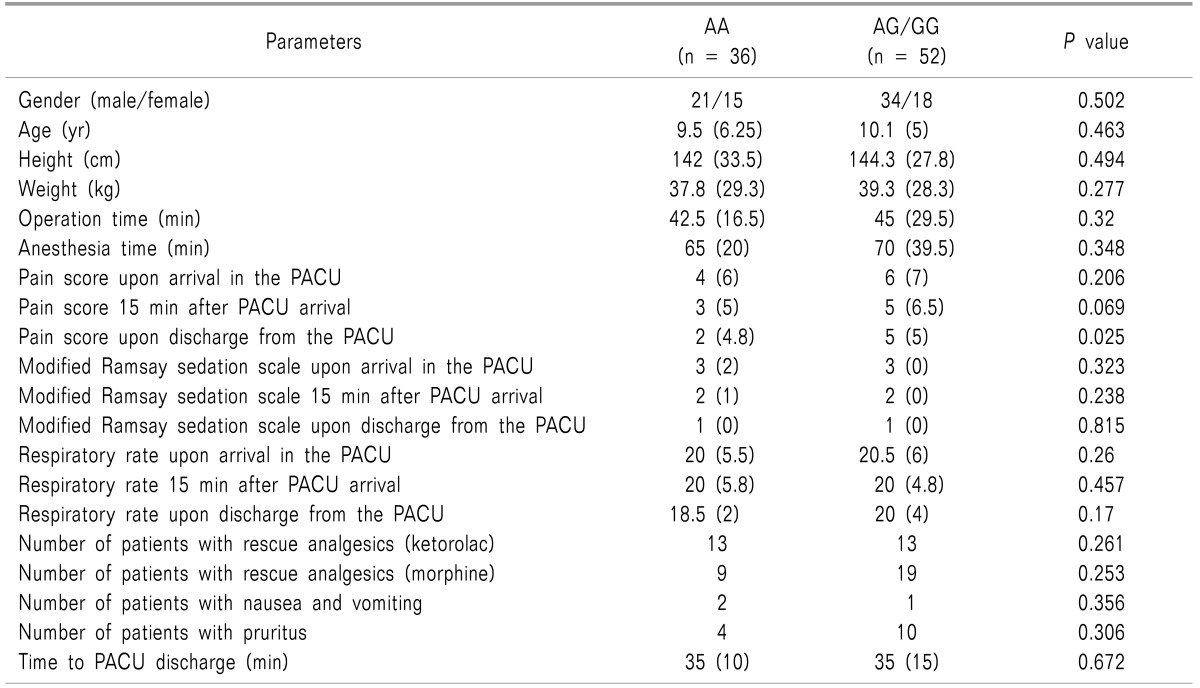
We evaluated A118G in relation to the postoperative pain score. Children with at least one G allele (AG/GG) had higher postoperative pain scores at the time of discharge from the PACU compared with those with the AA genotype (P = 0.025) (Fig. 1). Demographic data and other recovery profiles were not significantly different between the two groups (Table 3). Most side effects of morphine were mild and did not require intervention, and no patient experienced respiratory depression. No significant relationship was observed between genotypes and the postoperative pain score in analysis of C3435T and Val158Met polymorphisms.
Go to : 
Although morphine is a widely used medication for postoperative pain control in children, the analgesic effects have a large inter-individual variability. Recently, several genetic factors have emerged as subjects of major interest for understanding this variability in America and Europe [61314]. Because genetic factors are considerably influenced by ethnicity, the present study has special meaning in that the subjects are Korean children.
The OPRM1 A118G is one of the most well-known SNPs for modulating the analgesic response to morphine. This polymorphism leads to an exchange from asparagine to aspartate at position 40 of the mu-opioid receptor, thus deleting the N-linked glycosylation site in the extracellular receptor region [1516]. Additionally, the mutant receptor has a defect in mRNA transcription and protein yield. These changes alter the functional properties of the receptor for opioids and lead to variability in the analgesic effect of opioids [17]. The frequency of the minor allele (118G) has been reported variously from 0.01 to 0.5 according to ethnicity [1618]. It appears to be relatively high in Asian populations, and our study reveals the frequency as 0.375.
As a primary target for opioids, the mu-opioid receptor plays a key role in pain perception. Numerous studies have evaluated the association of OPRM1 A118G and postoperative pain in adults. Most of the studies have demonstrated that patients with the 118G variant required higher morphine doses for postoperative pain control [345819]. Some studies have reported that patients with the 118G variant recovered from anesthesia more quickly and experienced fewer of the side effects of opioids, such as respiratory depression and nausea/vomiting, than 118AA carriers [3420].
Little literature exists regarding the effect of the A118G SNP on postoperative pain in children. Chidambaran et al. [13] reported that the presence of the G allele was associated with higher postoperative pain scores and less respiratory depression during the 48 hours following spinal fusion in adolescents. Mamie et al. [14] also reported that children with the G allele experienced more postoperative pain compared with those with the AA genotype during the 24 hours after orthopedic or abdominal surgery. Both of the above studies [1314] examined postoperative pain and respiratory depression during relatively long postoperative periods in children who underwent major surgery and were administered patient-controlled morphine analgesia.
We investigated postoperative pain and recovery profiles in the PACU in children who underwent minor surgery without patient-controlled analgesia. As a result, we found that the A118G variant was also associated with immediate acute postoperative pain in children. However, we could not find any difference in recovery profiles such as respiratory rates according to the genotypes. This is probably because of the different subjects and methods of morphine administration. In fact, the overall incidence of respiratory depression reached 31% in the study of Chidambaran et al. [13], whereas no patients experienced respiratory depression in our study.
The P-glycoprotein is a type of integral membrane protein, which is encoded by ABCB1. Its function is the energy-dependent export of substances from the inside of cells to the outside. It is located in various organs as well as the blood-brain barrier, and influences the uptake of opioids into the brain. The ABCB1 C3435T is associated with reduced expression of P-glycoprotein [21]. Therefore, patients with the mutant genotype could have higher concentrations of morphine in the cerebrospinal fluid and require smaller doses of morphine for pain control [22].
The COMT Val158Met is another SNP of interest for postoperative pain management. COMT is an enzyme that metabolizes catecholamines such as adrenaline, noradrenaline, and dopamine, which are implicated in the modulation of pain. The Val158Met variant has been found to decrease the activity of the enzyme, leading to increases in catecholamine levels with rises in pain [23].
In the present study, we were unable to find a significant relationship between genotypes and the postoperative pain score in analysis of ABCB1 and COMT polymorphisms. Previous studies have shown inconsistent results regarding this issue. Mamie et al. [14] reported that children with the ABCB1 CC genotype experienced more postoperative pain compared with those with the CT/TT genotypes following orthopedic or abdominal surgery. Sadhasivam et al. [6] reported that, among children undergoing adenotonsillectomy, those with the COMT minor allele required more analgesic intervention than those who were homozygotes for major alleles. However, another recent study in Latino and non-Lation children found no difference in response to morphine in genetic analysis including OPRM1, ABCB1, and COMT [24]. This discrepancy may be caused by differences in the sample size and ethnicity of the study population. Additional research is needed to clarify these issues.
A possible limitation of this study is the high tendency of emergence delirium in children, which could disrupt accurate measurement of the pain score in the PACU. However, we injected morphine just before the end of the operation, and this could reduce the incidence of emergence delirium. In fact, the median modified Ramsay sedation scale score upon arrival in the PACU was 3 in both the AA and AG/GG groups. Another limitation is that the postopertive pain score was assessed only in the PACU. As postoperative pain could last for days, observation after PACU discharge might provide more information.
In conclusion, genetic polymorphism at OPRM1 A118G, but not at ABCB1 C3435T and COMT Val158Met, influences the analgesic effect of morphine for immediate acute postoperative pain in children. More research is needed to confirm this result and to improve our clinical practice based on genetic variations.
Go to : 
ACKNOWLEDGEMENTS
This work was supported by a research grant of Jeju National University in 2014.
Go to : 
References
1. Association of Paediatric Anaesthetists of Great Britain and Ireland. Good practice in postoperative and procedural pain management, 2nd edition. Paediatr Anaesth. 2012; 22(Suppl 1):1–79.
2. Dahmani S, Dupont H, Mantz J, Desmonts JM, Keita H. Predictive factors of early morphine requirements in the post-anaesthesia care unit (PACU). Br J Anaesth. 2001; 87:385–389. PMID: 11517121.

3. Wu WD, Wang Y, Fang YM, Zhou HY. Polymorphism of the micro-opioid receptor gene (OPRM1 118A>G) affects fentanyl-induced analgesia during anesthesia and recovery. Mol Diagn Ther. 2009; 13:331–337. PMID: 19791836.
4. Oertel BG, Schmidt R, Schneider A, Geisslinger G, Lötsch J. The mu-opioid receptor gene polymorphism 118A>G depletes alfentanil-induced analgesia and protects against respiratory depression in homozygous carriers. Pharmacogenet Genomics. 2006; 16:625–636. PMID: 16906017.

5. Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006; 105:334–337. PMID: 16871067.

6. Sadhasivam S, Chidambaran V, Olbrecht VA, Esslinger HR, Zhang K, Zhang X, et al. Genetics of pain perception, COMT and postoperative pain management in children. Pharmacogenomics. 2014; 15:277–284. PMID: 24533707.

7. De Gregori M, Garbin G, De Gregori S, Minella CE, Bugada D, Lisa A, et al. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur J Clin Pharmacol. 2013; 69:1651–1658. PMID: 23686330.

8. Hajj A, Peoc'h K, Laplanche JL, Jabbour H, Naccache N, Abou Zeid H, et al. Genotyping test with clinical factors: better management of acute postoperative pain? Int J Mol Sci. 2015; 16:6298–6311. PMID: 25809606.

9. Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997; 23:293–297. PMID: 9220806.
10. Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974; 2:656–659. PMID: 4835444.

11. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995; 7:89–91. PMID: 7772368.

12. Lötsch J. Basic genetic statistics are necessary in studies of functional associations in anesthesiology. Anesthesiology. 2007; 107:168–169. PMID: 17585231.

13. Chidambaran V, Mavi J, Esslinger H, Pilipenko V, Martin LJ, Zhang K, et al. Association of OPRM1 A118G variant with risk of morphine-induced respiratory depression following spine fusion in adolescents. Pharmacogenomics J. 2015; 15:255–262. PMID: 25266679.

14. Mamie C, Rebsamen MC, Morris MA, Morabia A. First evidence of a polygenic susceptibility to pain in a pediatric cohort. Anesth Analg. 2013; 116:170–177. PMID: 23223113.

15. Oertel BG, Kettner M, Scholich K, Renné C, Roskam B, Geisslinger G, et al. A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem. 2009; 284:6530–6535. PMID: 19116204.

16. Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998; 95:9608–9613. PMID: 9689128.

17. Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005; 280:32618–32624. PMID: 16046395.

18. Ono T, Muto A, Kaneda T, Arita E, Yoshida T. Novel linkage disequilibrium of single nucleotide polymorphisms in the transcriptional regulatory region of micro-opioid receptor gene in Japanese population. Biol Pharm Bull. 2009; 32:721–723. PMID: 19336912.

19. Kim KM. Opioid pharmacogenetics in anesthesia and pain management. Anesth Pain Med. 2015; 10:65–76.

20. Lee SH, Kim JD, Park SA, Oh CS, Kim SH. Effects of µ-Opioid receptor gene polymorphism on postoperative nausea and vomiting in patients undergoing general anesthesia with remifentanil: double blinded randomized trial. J Korean Med Sci. 2015; 30:651–657. PMID: 25931799.

21. Brinkmann U. Functional polymorphisms of the human multidrug resistance (MDR1) gene: correlation with P glycoprotein expression and activity in vivo. Novartis Found Symp. 2002; 243:207–210. PMID: 11990778.

22. Meineke I, Freudenthaler S, Hofmann U, Schaeffeler E, Mikus G, Schwab M, et al. Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br J Clin Pharmacol. 2002; 54:592–603. PMID: 12492606.

23. Berthele A, Platzer S, Jochim B, Boecker H, Buettner A, Conrad B, et al. COMT Val108/158Met genotype affects the mu-opioid receptor system in the human brain: evidence from ligand-binding, G-protein activation and preproenkephalin mRNA expression. Neuroimage. 2005; 28:185–193. PMID: 16040257.

24. Jimenez N, Anderson GD, Shen DD, Nielsen SS, Farin FM, Seidel K, et al. Is ethnicity associated with morphine's side effects in children? Morphine pharmacokinetics, analgesic response, and side effects in children having tonsillectomy. Paediatr Anaesth. 2012; 22:669–675. PMID: 22486937.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download