Abstract
Background
The purpose of this study was to evaluate the effect of dexamethasone or dexmedetomidine added to ropivacaine on the onset and duration of ultrasound-guided axillary brachial plexus blocks (BPB).
Methods
Fifty-one ASA physical status I-II patients with elective forearm and hand surgery under axillary brachial plexus blocks were randomly allocated to receive 20 ml of 0.5% ropivacaine with 2 ml of isotonic saline (C group, n = 17), 20 ml of 0.5% ropivacaine with 2 ml (10 mg) of dexamethasone (D group, n = 17) or 20 ml of 0.5% ropivacaine with 2 ml (100 µg) of dexmedetomidine (DM group, n = 17). A nerve stimulation technique with ultrasound was used in all patients. The onset time and duration of sensory blocks were assessed.
Go to : 
Axillary brachial plexus block (BPB) is commonly used for forearm and hand surgeries with ease, reliability, and a very low complication rate. To increase the duration of local anesthetic action, epinephrine, α2 agonist, corticosteroids, bicarbonate, and opioids have been used. Among these agents, epinephrine is most frequently added to local anesthetics. Epinephrine reduces absorption of local anesthetics. Therefore, it reduces the local anesthetics' systemic toxicity and prolongs anesthetic duration. In addition, it can aid early detection of intravascular injections. However, it can cause hypertension and tachycardia. Therefore its usage is limited when the patients have cardiovascular disease or hyperthyroidism.
Dexmedetomidine is a potent selective alpha-2 agonist of which α2/α1 selectivity is 8 times greater than clonidine. A recent study reported dexmedetomidine to the axillary BPB prolonged the duration of sensory and motor nerve blocks without any significant complications [1].
However, there has been no research conducted to compare the effects of dexamethasone and dexmedetomidine added as adjuvants to the local anesthetics for axillary BPB. Therefore, we conducted a study to compare the onset and duration of ropivacaine action when dexmedetomidine or dexamethasone is used as an adjuvant to ropivacaine for ultrasound-guided axillary BPB.
Go to : 
This was a prospective, randomized, double-blind study conducted after Institutional Review Board approval was obtained. Written informed consent was obtained from 75 American Society of Anesthesiologist (ASA) Grade I and II patients more than 18 years of age who were scheduled for elective hand and forearm surgery under axillary BPB. Patients with hypertension, cardiac or hepatic diseases, diabetes mellitus, a coagulopathy, a history of taking α-2 adrenergic drugs, or an infection in the puncture site were excluded from the study.
No patients received premedication. The computer randomly assigned patients to the control group (C group, n = 17), the dexamethasone group (D group, n = 17), or the dexmedetomidine group (DX group, n = 17) just before arrival in the operating room. After arrival in the operation room, all patients were monitored with electrocardiogram, non-invasive blood pressure and pulse oxymetry. Axillary brachial plexus blocks were performed with the patient in the supine position and the upper arm abducted 90° and the elbow flexed at 110°. After injecting of 3 ml of 2% lidocaine for anesthetizing the skin, a 22-gauge, 50 mm short beveled needle (Stimulex®A, B/Braun, Melsungen, Germany) and nerve stimulator (Stimulex® Dig RC, B/Braun, Melsungen, Germany) were used to examine the nerve with ultrasound (Sonosite®, Bothell, WA, USA). At first, motor response was sought by stimulating with a 0.8 to 1.0 mA current intensity and a frequency 1 Hz. When the proper twitch was elicited, stimulating intensity was gradually decreased to less than 0.5 mA. Once the proper twitch was maintained with a current less than 0.5 mA, 1 ml of local anesthetics was injected. After this injection stopped the twitch, the position of the needle was considered to be acceptable, and the remaining 4.5 ml was injected. All four branches were blocked separately with 5.5 ml of 0.5% ropivacaine (Ropiva®, Hanlim, Korea) (total amount of 22 ml) according to the study group. Group C was administered 2 ml of normal saline. Group D was administered 2 ml of 10 mg dexmedetomidine (Dexamethasone®, Yuhan, Korea). Group DX was administered a 2 ml of mixture containing 1 ml of normal saline and 1 ml of 100 µg of dexmedetomidine (Precedex®, Hospira, USA) 1 ml. In addition, 20 ml of 0.5% ropivacaine was added to all groups to achieve a total of 22 ml. A blinded observer recorded the time used to perform the block, measured from localization of the indivisual nerves to completion of local injection of anesthetics.
The degree of sensory block was assessed using pinpricks in the each of nerve distributions of the musculocutaneous nerve (forearm), radial nerve (dorsal 1st and 2nd intermetacarpal area), median nerve (palmar side of tip of the 3rd finger), and ulnar nerve (palmar side of tip of the 5th finger) and was graded according to a two-grade scale (0 = no block, 1 = loss of pinprick sensation). The degree of sensory block was evaluated every 2 minutes after drug administration for 30 minutes. Onset time was defined as the time interval between the end of local anesthetic injection and the loss of pinprick sensation and a successful block was defined as grade 1 sensory block. The duration of the sensory nerve block was considered to be the time interval between a successful block and the complete restoration of all the senses controlled by the radial, ulnar, median and musculocutaneous nerves.
The quality of anesthesia was measured by the need for supplemental opioids during the operation. When pain more than 4 on visual analogue scale (VAS; 0 = no pain, 10 = worst pain imaginable) or an uncomfortable sensation developed during surgery, a 50 µg bolus of fentanyl was administered intravenously. If pain persisted 5 minutes after administration of fentanyl, an additional 50 µg fentanyl was given. When less than 100 µg of fentanyl was needed to complete surgery, the block was considered a "sufficient block". When more than 100 µg of fentanyl was needed to complete surgery, the block was considered an "insufficient block". If fentanyl supplementation was not sufficient to complete surgery, the block was considered a "failed block" and was converted to general anesthesia [4]. Adverse events included hypotension (a 20% decrease of base blood pressure), bradycardia (HR < 50 beats/min), hypoxemia (SpO2 < 90%), or nausea and vomiting. Glycopyrrolate 0.2 mg or ephedrine 4 mg was intravenously administered.
The duration of the sensory block was the primary outcome. In a pilot study conducted with 15 patients (5 in each group), assuming that 100 is the maximum standard deviation, the sample size necessary for a 0.8 statistical power and α value 0.05 was calculated to be 17 per group. The data were expressed as mean ± standard deviation or number (%). Age, height, weight, duration of surgery, duration of the tourniquet, time to perform the block, onset time and duration of sensory block were compared among among the 3 groups by a one-way ANOVA and Tukey post hoc tests. And the sex and ASA physical status were compared using the chi-square test and Fisher's exact test. Statistical analysis was performed using IBM SPSS 21.0 (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered significant.
Go to : 
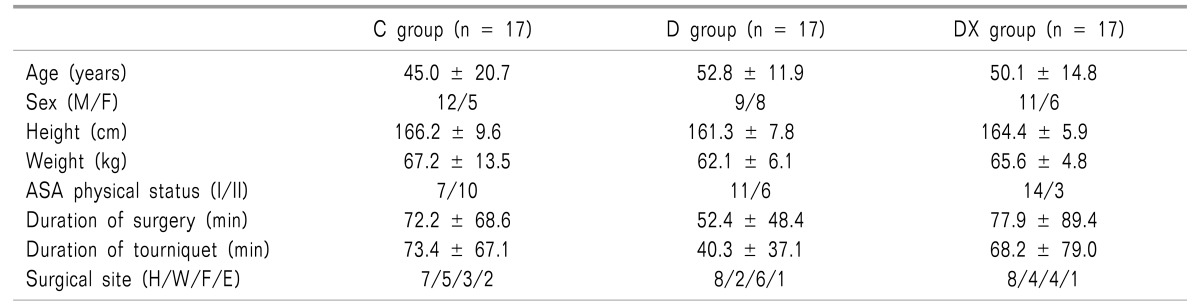
There were no significant differences between the three groups in demographic data (Table 1). The onset time of the sensory block showed no significant difference between the three groups (Table 2). The duration of the sensory block was extended in group D and group DX compared to group C (P < 0.05), but there was no significant difference between group D and group DX (Table 2). There was no significant difference in the quality of the blockade between the groups. One patient in group D needed 50 µg of fentanyl. And no failed block was reported in any of the groups (Table 2). In the DX group, bradycardia was observed in 1 patient, and this patient was treated with glycopyrrolate. Adverse reactions, including hypotension, hypoxemia, or nausea/vomiting were not reported in all 3 groups.
Go to : 
In this study, we demonstrated that both dexamethasone and dexmedetomidine, added to ropivacaine, extended block duration compared to the control group in patients undergoing ultrasound-guided axillary BPB. But, there were no significant differences between dexamethasone and dexmedetomidine groups. In addition, there were no significant differences in onset time among the 3 groups. This is generally consistent with other previous reports [12]. However, Esmaoglu et al. [5] reported that dexmedetomidine decreased the onset time in axillary BPB. They used 40 ml of 0.5% levobupivacaine and a nerve stimulator without ultrasound. The equivocal results can be assumed to result from differences of local anesthetics, dosage, and methods of access to the brachial plexus. Further studies should attempt to verify these assumptions.
By using ultrasound in real time together with a nerve stimulator, appropriate spread of local anesthetics was ascertained using a relatively smaller volume (22 ml total). So, we could succeed with the nerve block in all patients without any complications.
The mechanism of dexamethasone in prolonging the duration of nerve blocks is not completely understood and is thought to arise from various factors.
Possible explanations may be related to a degree of vasoconstriction which results in the absorption of local anesthetics [6], suppression of the synthesis and secretion of inflammatory mediators [7], reducing the transmission in unmyelinated C-fiber [8].
The safety of dexamethasone within the nerve sheath may raise some concerns because it is not an indication of this drug. Several studies have reported about the safety of corticosteroid [91011]. In Sugita's study, repeated intrathecal injection of dexamethasone 8 mg once or twice a week (total dose 80-100 mg) to treat posttraumatic visual disturbances in 200 patients caused no serious complications [9]. Therefore, it seems to be safe to use a single dose of dexamethasone. However, it can raise glucose and act as a potent immunosuppressant. So, it is not preferable to use with diabetics or patients with severe infections.
The mechanism of the analgesic effect of dexmedetomidine is not fully understood and it is likely to be multifactorial.
Possible explanations may be related to a vasoconstriction property [12], altering locus ceruleus activity and a decrease in release of norepinephrine from the locus coeruleus [13]. Several studies have reported the safety and effectiveness of dexmedetomidine as an adjuvant to local anesthetics in spinal and Bier's blocks [141516]. Brummett et al. [17] reported perineural administration of a large dose dexmedetomidine to enhance bupivacaine duration without inducing neuron toxicity in rats. Dexmedetomidine can cause side effects such as bradycardia and hypotension with an increased dose. In our study, bradycardia occurred in one patient. Several studies reported high incidences of bradycardia [1518]. It can be easily controlled in healthy patients by using glycopyrrolate.
However, dexmedetomidine administration should be carefully conducted in patients with a history of cardiac disease, especially clinically significant arrhythmia.
In conclusion, dexamethasone and dexmedetomidine have similar effects on extending the duration of ropivacaine in ultrasound-guided guided axillary BPB. However, neither drug has significant effect on the onset time.
Go to : 
References
1. Song JH, Shim HY, Lee TJ, Jung JK, Cha YD, Lee DI, et al. Comparison of dexmedetomidine and epinephrine as an adjuvant to 1% mepivacaine in brachial plexus block. Korean J Anesthesiol. 2014; 66:283–289. PMID: 24851163.

2. Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg. 2006; 102:263–267. PMID: 16368840.

3. Kim YJ, Lee GY, Kim DY, Kim CH, Baik HJ, Heo S. Dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean J Anesthesiol. 2012; 62:130–134. PMID: 22379567.

4. Kim W, Kim YJ, Kim JH, Kim DY, Chung RK, Kim CH, et al. Clinical comparisons of 0.5% and 0.375% levobupivacaine for ultrasound-guided axillary brachial plexus block with nerve stimulation. Korean J Anesthesiol. 2012; 62:24–29. PMID: 22323950.

5. Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010; 111:1548–1551. PMID: 20889939.

6. Marks R, Barlow JW, Funder JW. Steroid-induced vasoconstriction: glucocorticoid antagonist studies. J Clin Endocrinol Metab. 1982; 54:1075–1077. PMID: 7061698.

7. Bastos LF, Medeiros DC, Vieira RP, Watkins LR, Coelho MM, Moraes MF. Intraneural dexamethasone applied simultaneously to rat sciatic nerve constriction delays the development of hyperalgesia and allodynia. Neurosci Lett. 2012; 510:20–23. PMID: 22240103.

8. Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990; 34:335–338. PMID: 2167604.

9. Sugita K, Kobayashi S, Yokoo A, Inoue T. Intrathecal steroid therapy for post-traumatic visual disturbance. Neurochirurgia (Stuttg). 1983; 26:112–117. PMID: 6688660.

10. Abram SE, Marsala M, Yaksh TL. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994; 81:1198–1205. PMID: 7978478.

11. De Oliveira GS Jr, Castro Alves LJ, Nader A, Kendall MC, Rahangdale R, McCarthy RJ. Perineural dexamethasone to improve postoperative analgesia with peripheral nerve blocks: a meta-analysis of randomized controlled trials. Pain Res Treat. 2014; 2014:179029. PMID: 25485150.

12. Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003; 99:65–70. PMID: 12826844.

13. Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996; 84:873–881. PMID: 8638842.

14. Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006; 50:222–227. PMID: 16430546.

15. Memiş D, Turan A, Karamanlioğlu B, Pamukçu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004; 98:835–840. PMID: 14980948.
16. Esmaoglu A, Mizrak A, Akin A, Turk Y, Boyaci A. Addition of dexmedetomidine to lidocaine for intravenous regional anaesthesia. Eur J Anaesthesiol. 2005; 22:447–451. PMID: 15991508.

17. Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008; 109:502–511. PMID: 18719449.

18. Kim KH. Safe sedation and hypnosis using dexmedetomidine for minimally invasive spine surgery in a prone position. Korean J Pain. 2014; 27:313–320. PMID: 25317279.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download