Abstract
Background
The present study was undertaken to evaluate the incidence of chronic persistent post-surgical pain (CPPP) and the role of signal transduction genes in patients undergoing staging laparotomy for carcinoma ovary.
Methods
The present observational study was undertaken following institutional ethical committee approval and informed consent from all the participants. A total 21 patients of ASA grade I to III with age 20−70 years, scheduled for elective staging laparotomy for carcinoma ovary were included. Patients were excluded if had other causes of pain, cognitive dysfunction or chronic neurological disorders. Statistical analysis of pool data was done using SPSS version-17. For various scales like GPE, PDQ, NPSI, the visual analogue scale (VAS), global perceived effect (GPE), the pain DETECT questionnaire (PDQ), and neuropathic pain symptoms inventory (NPSI), one factor repaeted measure ANOVA applied with simple contrast with baseline as on post-operative day 1 (considered as reference and compared with subsequent time-interval), and the P values were adjusted according to "Bonferroni adjustments". In patients with CPPP, the Δct values of mRNA expressions of genes at the end of postoperative day 90 were compared with the baseline control values by one factor repeated ANOVA. P value < 0.005 significant.
Results
The present study demonstrates 38.1% (8 out of 21 patients) incidence of CPPP. The functional status and quality of life as were observed to be significantly diminished in all patients with chronic pain. An up-regulation in the mRNA expression of signal transduction and a positive correlation was noted between the mRNA expression of signal transduction genes and VAS score in all patients with CPPP at the end of postoperative day 90.
Go to : 
Chronic persistent post-surgical pain (CPPP) is a commonly encountered complication following surgery and has a significant humanitarian and socio-economic burden [12]. The overall reported incidence after various common surgical procedures ranges between 5-50% [1]. The incidence of CPPP varies amongst various types of surgery: from inguinal herniorraphy to thoracotomy to breast surgery [1]. Intra-operative nerve injury induced neuropathic pain has repeatedly been proposed as a major cause of CPPP [13].
Hysterectomy is a frequent gynaecological surgery and the reported prevalence of chronic pain following hysterectomy is 5%-32% [45]. Most of the prospective studies examining the incidence of CPPP following hysterectomy are for benign conditions [6]. On searching the literature, we could not find any article reporting the incidence and severity of CPPP following staging explorative laparotomy for any gynecological carcinomas including ovarian carcinoma.
Genetic predisposition is an important predictive preoperative risk factor for development of CPPP [78]. In the last two decades, it has been suggested that peripheral nerve injury induces marked molecular changes in gene expression in both the dorsal root ganglia and dorsal horn of the spinal cord contributing to the development of neuropathic pain [9]. Various animal studies have highlighted the contribution of genes encoding second messenger molecules, that are found in the substantia gelatinosa like the proteins kinase A, C, nitric oxide synthase, and ERK in the pathogenesis of neuropathic pain [910111213]. However, no human study exists pertaining to the role of mRNA expression of signal transduction genes in the genesis of chronic persistent post surgical pain.
So the present study was undertaken to report the incidence and severity of CPPP in patients undergoing staging explorative laparotomy for ovarian carcinoma and to study the contribution of genes encoding major second messenger molecules like protein kinase A, protein kinase C and ERK, in the development of CPPP in these patients.
Go to : 
This cross-sectional follow-up study was undertaken following approval from the Institutional Ethical Committee for Human Research. The study was conducted in the departments of Anaesthesiology and Critical Care, Biochemistry, and Obstetrics & Gynaecology. All female patients were of American Society of Anaesthesilogists (ASA) grades I–III, who were scheduled for elective staging exploratory laparotomy (hysterectomy, bilateral salpingo-oophorectomy, omentectomy and lymphadenectomy) for ovarian carcinoma with a midline incision (may extend above the umbilicus) were included in the study. Surgeries were conducted by the surgeons with expertise in gynaecological oncology-surgery. Patients were excluded from the study if their ASA grade was IV and V, if they had other causes of pain, had cognitive dysfunction, or any chronic neurological disorders.
A written informed consent was taken from each patient included in the study. The patients were informed that following surgery, they would be followed up for a minimum period of 4 months, which would include a detailed evaluation and assessment of pain at various designated intervals. In the preoperative period, all patients were assessed for their predisposition to development of chronic pain by enquiring about any complaints of generalized pain syndrome like headache, low back pain, musculoskeletal pain, or a tendency for exaggerated and pain at a site other than the pathologic site. Also, a detailed psychological and functional status evaluation of the patients was done in the preoperative period, using the Hospital Anxiety and Depression Scale (HADS) and Activity Assessment Scale (AAS), respectively. The functional status of the patient was assessed using AAS with a 12-point questionnaire at varying intervals i.e. preoperatively, and postoperatively, on day 3, day 60, day 90, and day 120. The AAS involves a set of 12 questions to assess the functional activity of the patient, during the past 24 hours and each activity is scaled from 1-5 i.e., from "no difficulty" to "not able to do". If the patient did not perform that act due to some other reason, a score of 8 is given. A baseline 2 ml venous sample was collected in an EDTA vial and stored at −20 degree Celsius after fixing it in TRIZOL reagent for further gene expression studies.
A standard anesthetic technique was adopted in all the patients. The technique included preoperative thoracic epidural catheter insertion followed by the administration of general anaesthesia (GA). The epidural catheter was inserted under aseptic conditions with the patient in a sitting position, using the classical technique eliciting loss of resistance. A multimodal approach for pain management was adopted for postoperative pain management for 72 hrs. It encompassed epidural analgesia including Bupivacaine with fentanyl and systemic analgesia including IV paracetamol three times a day and IV diclofenac twice a day.
In the post-operative period, a detailed evaluation of the intensity and quality of pain was done using five different questionnaires at varying intervals, i.e., at the end of postoperative day 1, day 3, day 14, day 30, day 60, day 90 and day 120. The visual analogue scale (VAS), numerical rating scale-sleep (NRS-Sleep) and global perceived effect (GPE) were used for measuring the intensity and quality of pain at the aforementioned designated intervals. The intensity of pain was evaluated using the VAS pain score ranging from 0-10 i.e. no pain to worst pain imaginable. Pain was graded as severe if the VAS was ≥ 7/10 and moderate if it was 4/10-6/10. Whereas in the GPE, pain intensity is graded from 1-7, 1 being the worst ever and 7 being the best ever. Patients interference with sleep was evaluated using NRS-Sleep ranging from 0-10, 0 being no interference to 10 being complete interference. The neuropathic component of pain was assessed by using the pain DETECT questionnaire (PDQ) and neuropathic pain symptoms inventory (NPSI), again at the same designated time intervals. In the PDQ, the score is between 0-35, with 13-18 reflecting the possibility of a neuropathic component to the pain, which is considered highly likely if the score is more than 18. The neuropathic pain symptoms inventory (NPSI) scoring for various neuropathic sensations like "electric-shock" like pain, "stabbing", "tingling", "pins & needles", and "allodynia" were done and graded on a scale of 0-10. In case of unsatisfactory pain relief i.e. VAS ≥ 3/10, cap pregabalin 75 mg (bid) (with adequate interval of 10-12 hours between two consecutive doses) and tab. piroxicam 20 mg dispensable (bid) was started and continued till the VAS score was < 3/10. A record was maintained for all these patients.
The assessment of the quality of life and the functional status of all the patients in the postoperative period was done using short form 12 (SF-12) questionnaires and the activity assessment scale (AAS), respectively on post-operative day 3, day 60, day 90 and day 120. Quality of life was studied using the SF-12 questionnaire from the postoperative day 3 onwards; whereas, the baseline functional status was assessed using AAS preoperatively. SF-12 consists of two components i.e., the physical component summary (PCS) and the mental component summary (MCS). The PCS and MCS scores have a range of 0 to 100 and were designed to have a mean score of 50 and a standard deviation of 10 as per a representative sample of the US population. Thus, a score greater than 50 represents an above average health status. The pain experienced or perceived by the patients was labelled as chronic persistent postsurgical pain (CPPP) if the total duration of pain in the postoperative period was at least three months, and also if other causes of pain had been excluded e.g. chronic infection, continuing malignancy, etc.
For estimation of the PKA, PKC, and ERK gene expression study, following a baseline sample, a repeat 2 ml venous sample was collected at the end of postoperative day 3, day 30 and day 90. All the molecular biological studies were performed in a Laminar Air Cabinet (Toshiba, India). Estimation of PKA, PKC and ERK gene expression in the blood was performed using a CFX connect Biorad two color High Resolution Melt (HRM) Real Time PCR in the following steps:
RNA was isolated from whole blood using Trizol BD (Ambion, Invitrogen Bioservices, India) as per the manufacturer's protocol. The concentration and purity of the samples were determined using a spectrophotometer (Nanodrop1000; Thermofisher, USA). All the samples had a ratio of absorbance at 260/280 between 1.8 and 2.0.
From the total RNA extracted, mRNA was used as a template for the synthesis of cDNA. On the same day preferably, total RNA (1 µg) was converted into first strand cDNA using a Maxima first strand cDNA synthesis kit (Thermo USA) according to the manufacturer's protocol.
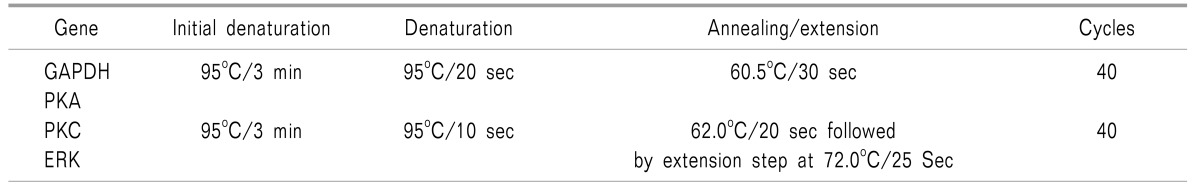
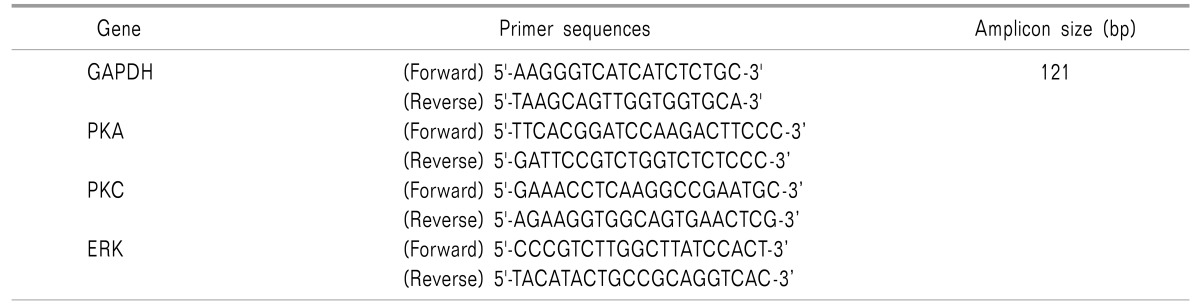
A real time quantitative polymerase chain reaction (RTqPCR) experiment was conducted to measure expression of PKA, PKC and ERK genes in blood samples from the subjects. The qPCR reactions were performed on CFX connect Biorad two colors High Resolution Melt (HRM) Real Time PCR, following PCR conditions and primer sequences. Tables 1 and 2 show the cycling conditions and the primer sequences of genes used for the Real-Time PCR assay.
Then, delta Ct was evaluated, which is the difference between the average Ct of the target gene (PKA, PKC and ERK gene) and a normalizer gene (GAPDH gene). Delta Ct value is inversely proportional to the nucleic acid content, and thus the lower the value, the higher the expression, and vice-versa.
Again, the difference of the mean Ct values of the control and cases were determined i.e. ΔΔCt.
The sample size could not be calculated but was determined on the basis of patient reporting to our institution as per the hospital records. Only 1-2 patients per month with ovarian carcinoma, on average, reported to our institution for staging laparotomy, and since this study was limited to 18 months, a minimum of 20 patients with ovarian carcinoma undergoing staging laparotomy could be included.
The statistical analysis of pool data was done using SPSS software (Version17). Descriptive statistics were used for summarization and calculation of the incidence of CPPP at postoperative day 90. To compare the changes in the VAS pain score, as well as the PDQ, NPSI, AAS, and SF-12 scores, at various designated intervals, one factor repeated measure ANOVA was applied. Simple contrast with the baseline as on post-operative day 1 (considered as a reference and compared with subsequent time-intervals), and the P values were adjusted according to "Bonferroni adjustments" (adjusted = original P value × number of comparisons). The "Friedman test" was applied to compare the distribution of repeated NRS-sleep scores (the Wilcoxan signed rank test was applied for multiple comparisons) and P values were adjusted according to the Bonferroni correction.
Δct value is inversely proportional to the nucleic acid content, so on the basis of Δct value and by applying one factor repeated ANOVA, the P value was calculated using baseline values as the control value and values of the blood samples drawn post-operatively on day 3, day 30 and day 90 as case values. In patients with CPPP, the Δct values of mRNA expressions of genes at the end of postoperative day 90 were compared with the baseline control values by one factor repeated ANOVA. The correlation between the mRNA expression of genes with VAS score at the end of day 3, day 30 and day 90 was done using the "spearman correlation". A P value less than 0.05 was considered significant.
Go to : 
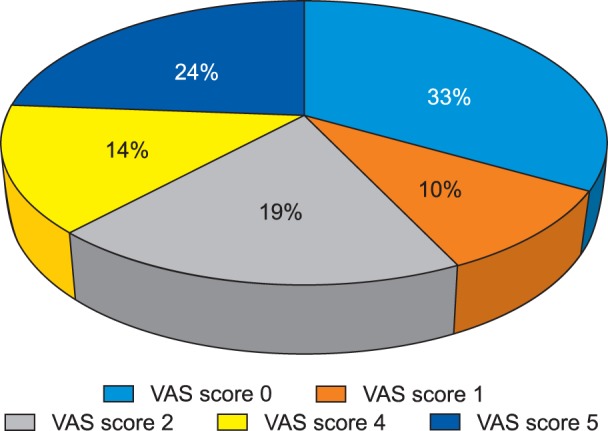
A total of 27 female patients undergoing staging laparotomy for ovarian carcinoma were included in this follow-up observational study. Out of the 27 patients; two had expired and four could not be followed up subsequently. Finally, a total of 21 patients were included in the study and were also followed up for the complete 4 months. The mean ± SD of the age of the patients and the duration of the surgery were 47.6 ± 14.7 years (22-67 years) and 185 ± 28 minutes, respectively. None of the patient gave any history of chronic pain. A detailed psychological evaluation of the patients was done using HADS in the preoperative period. All patients had normal HADS score, with a mean ± SD of 1.9 ± 1.70. The mean VAS score at the end of postoperative day 1, day 3, day 14, day 30, day 60, day 90 and day 120 were 6.3 ± 0.9, 6.1 ± 0.7, 4.7 ± 0.8, 3.7 ± 1.2, 2.8 ± 1.7, 2.3 ± 2.1 and 1.7 ± 1.9, respectively (Fig. 1). Statistically, no significant difference was found for the VAS at day 3; whereas, statistically significant differences (P < 0.05) were observed at the other designated intervals when compared to the first postoperative day. On the 1st and 3rd postoperative day, 47.6% (n = 10) and 33.3% (n = 7) patients had severe pain. On the 14th postoperative day, all 21 patients selected for survey had moderate pain and none of the patients had severe pain. On the 30th and 60th postoperative days, 90.5% (n = 19) and 38.1% (n = 8) patients had moderate pain, respectively and none of the patients had reported severe pain. On postoperative day 90, 38.1% (n = 8) of the patients had moderate pain, 28.6% (n = 6) had reported mild pain and 33.3% (n = 7) had no pain (Fig. 2).
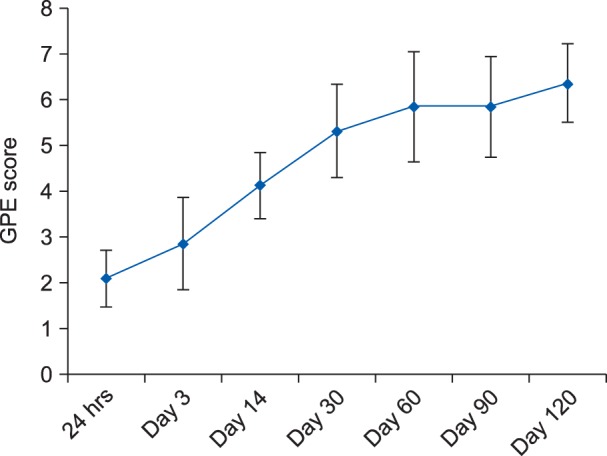
Similarly, on postoperative day 120, it was observed that 38.1% (n = 8) of patients had moderate pain, 19% (n = 4) had mild pain, and 42.9% (n = 9) had no pain. The mean ± SD of the GPE score at the end of day 1, day 3, day 14, day 30, day 60, day 90 and day 120 postoperatively were 2.1 ± 0.6, 2.8 ± 1.1, 4.1 ± 0.7, 5.3 ± 1.0, 5.8 ± 1.2, 5.9 ± 1.0 and 6.4 ± 0.8, respectively (Fig. 3). A statistically significant difference (P < 0.05) was observed with regards to the GPE scoring on postoperative day 3, day 14, day 30, day 60, day 90 and day 120 when compared to postoperative day 1.
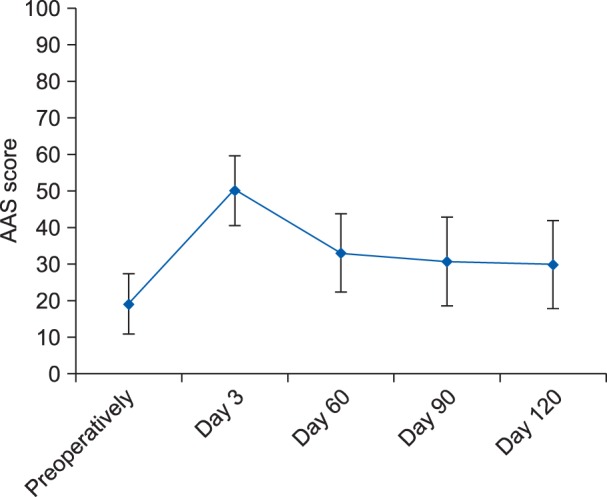
The mean ± SD of the AAS score preoperatively and then on postoperative day 3, day 60, day 90, and day 120 were 18.8 ± 8.3, 49.9 ± 9.5, 32.8 ± 10.7, 30.4 ± 12.2, and 29.7 ± 12.0, respectively (Fig. 4). When compared to the preoperative value, the AAS score was found to be statistically significant (P < 0.05) postoperatively at the end of day 3, day 60, day 90, and day 120 (Fig. 4). The median (IQR) of the NRS scores were 7 (6-7), 6 (5-6), 4 (3-5), 3 (1-4.5), 0 (0-4), 0 (0-4), and 0 (0-3.5) at the end of postoperative day 1, day 3, day 14, day 30, day 60, day 90, and day 120, respectively. No statistically significant difference was found for NRS-sleep score at day 3; whereas, statistically significant difference (P < 0.05) were observed at other designated intervals when compared to postoperative day 1.
An evaluation of the neuropathic component of the pain was done by PDQ scoring at designated intervals. The mean PDQ score on postoperative day 1, day 3, day 14, day 30, day 60, day 90 and day 120 postoperatively were 16.2 ± 2.2, 14.3 ± 1.7, 9.5 ± 4.5, 8.48 ± 5.192, 6.8 ± 6.3, 6.3 ± 6.6 and 5.6 ± 6.8, respectively. A statistically significant difference (P < 0.05) was observed when PDQ scoring at postoperative day 3, day 14, day 30, day 60, day 90, and day 120 were compared with postoperative day 1. NPSI scoring for various neuropathic sensations like "electric-shock" like pain, "stabbing", "tingling", "pins & needles", and "allodynia" were done on postoperative day 1, day 3, day 14, day 30, day 60, day 90, and day 120, and was graded on a scale of 0-10. The prevalence of the various types of neuropathic sensations at end of postoperative day 90 in patients with a VAS score ≥ 4/10 is shown in Fig. 5.
The quality of life was studied using the SF-12 questionnaire which consisted of two components i.e., the physical component summary (PCS) and the mental component summary (MCS). The average MCS score on the 30th day, 60th day, 90th day and 120th days are 35.3 ± 4.07, 43.0 ± 4.7, 49.8 ± 4.2, and 53.8 ± 4.4, respectively. Similarly, the mean PCS scores on the 30th, 60th, 90th, and 120th days are 34.7 ± 4.6, 42.9 ± 4.6, 48.4 ± 4.0, and 51.7 ± 3.3, respectively. A statistically significant (P < 0.05) increase in both the MCS and PCS components of the SF-12 was observed at postoperative day 60, day 90, and day 120 when compared to the baseline value on the postoperative day 30. The perioperative period remained uneventful in all the patients.
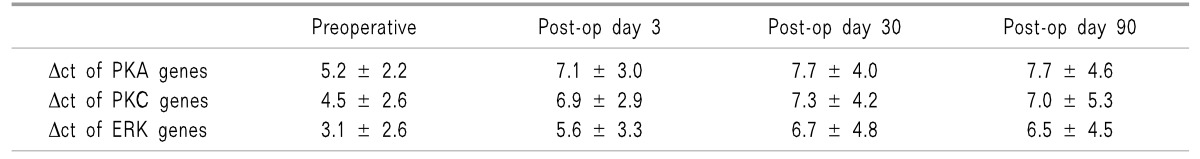
Δct value is inversely proportional to the nucleic acid content, thus higher values represent down-regulation of gene expression and vice-versa. Table 3 shows the mean Δct values of the PKA, PKC, and ERK genes in all 21 patients. There was a statistically significant down regulation in the mRNA expression of the PKA, PKC, and ERK genes at day 3, day 30, and day 90, postoperatively when compared to the preoperative baseline value.
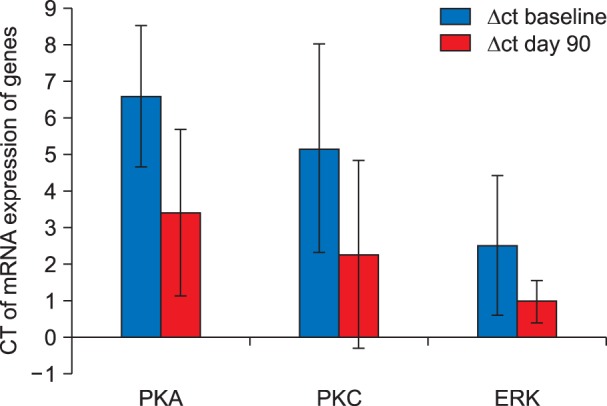
On Comparison of the preoperative Δct value of PKA, PKC and ERK with the post-operative Δct value of the mRNA expression of PKA, PKC, and ERK in the patients who complained of pain (VAS ≥ 4/10) at the end of postoperative day 90, a significant up-regulation in the mRNA expression of all the three signal transduction genes were observed (Fig. 6).
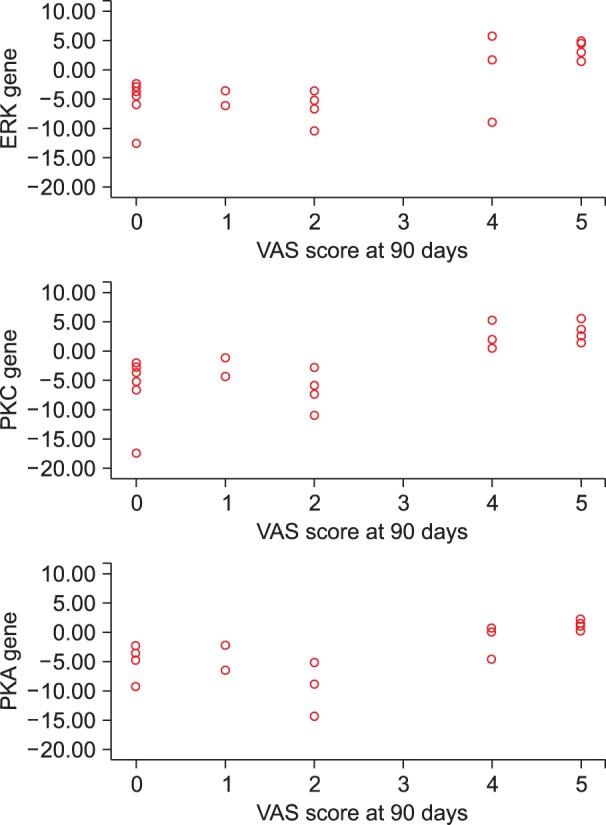
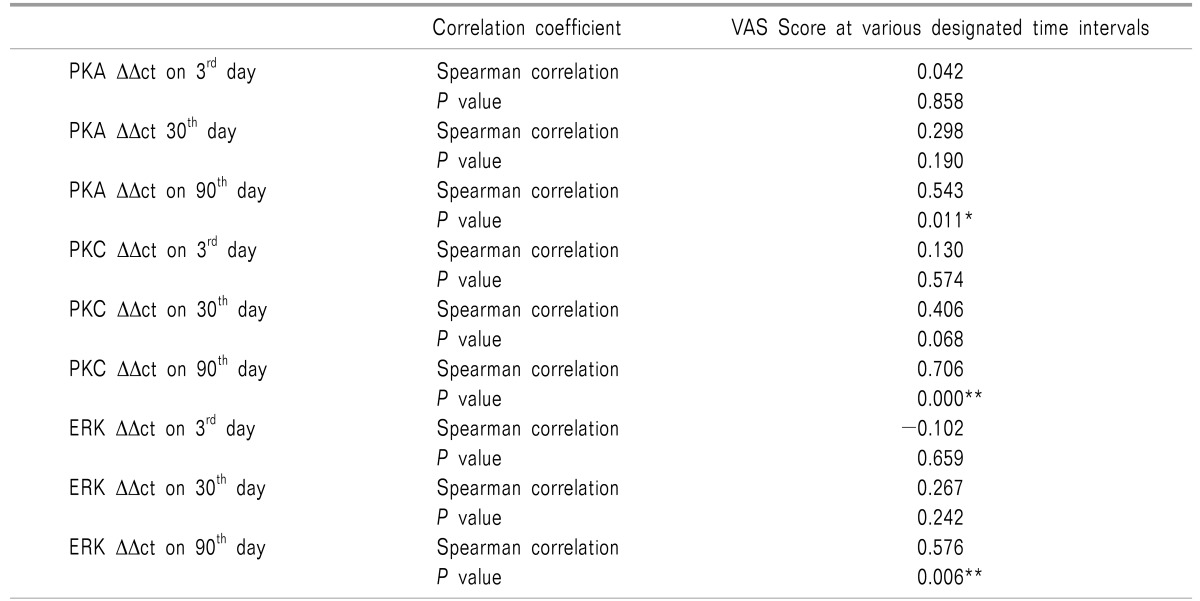
On correlating the VAS scores of patients complaining of pain at day 90 with the ΔΔct values of PKA, PKC and ERK at various designated intervals i.e. the 3rd day, 30th day, and 90th day; a significant positive correlation was observed on postoperative day 90 (Table 4, Fig. 7).
Go to : 
The present follow-up observational study demonstrates a 38.1% (8 patients with VAS ≥ 4/10 out of 21 patients) incidence of chronic persistent surgical pain at postoperative day 90 in patients undergoing staging laparotomy for ovarian carcinoma. At the end of postoperative day 90, all patients with CPPP had moderate pain, i.e. a VAS score of 4/10-6/10. Interestingly, these patients were also observed to have up-regulation in the mRNA expression of signal transduction genes i.e. PKA, PKC and ERK. Also, a positive correlation was observed between CPPP and the mRNA expression of PKA, PKC, and ERK genes.
After extensive search in PubMed and Medline, it was noted that the incidence of chronic persistent post-surgical pain following staging laparotomy has never been explored for any type of gynaecological carcinoma including ovarian carcinoma. Various researchers have studied the incidence of chronic pain following a total abdominal hysterectomy (TAH) which were observed to be around 5%-32% [451415]. Hence, in comparison to the reported data of CPPP following TAH, the higher incidence (i.e. 38.1%) in the current study involving patients undergoing staging laparotomy for ovarian carcinoma, could be attributed to the use of a larger midline incision (vis-a-vis the pfannelstiel incision in TAH) and extensive tissue handling probably contributing to nerve injury.
Most importantly, the severity of acute post-operative pain is an important determinant for development of CPPP [161718]. Multimodal analgesic technique and pre-emptive analgesia have been found to have preventive roles in regard to chronic persistent postsurgical pain [19]. Obata et al. [20] and Gottschalk et al. [21] observed that peri-operative use of epidural analgesia for thoracotomy and laparotomy reduces the incidence of CPPP. In the present survey, as an institutional protocol, multimodal analgesia using epidural analgesia along with IV morphine, diclofenac, or paracetamol was administered to all the patients. Despite the use of epidural analgesia in the post-operative period for 72 hours in all the patients, 47.6% and 33.3% of patients had severe pain on postoperative day 1 and 3, respectively. This higher incidence could be attributed to the use of epidural top-ups only, rather than continuous epidural infusion in the current study due to the non-availability of syringe infusion pumps connected to epidural catheters and patient controlled analgesia (PCA) sets in our institute. For rescue analgesia, a combination of pregabalin and piroxicam was used as they take care of the "neuropathic" and "nociceptive" components of persistent postsurgical pain.
Preoperative pain at the site of surgery [1422], as well as pain problems elsewhere are important predictive factors for development of CPPP [1423242526]. In the present study, the patient population involved had ovarian carcinoma which is usually not associated with pain. Moreover, none of the patients in the current series had preoperative pain of any etiology. Despite the absence of risk factors like preoperative pain and pain of any etiology in our study population, we observed a high incidence and severity of CPPP.
Nerve injury-induced neuropathic pain has been considered to be the main pathogenic mechanism for the development of CPPP [13]. Assessment of the neuropathic component of post-surgical pain is essential to plan effective strategies for its prevention and treatment. A lack of uniformity in neuropathic pain assessment has been observed across various studies. To date, the validated tools that have been used to assess the neuropathic pain syndrome of both central and peripheral origins are the NPSI [27] and PDQ [28], and in the current study they have been used extensively at designated intervals. On review of the literature, it was observed that a majority of studies evaluating chronic pain following TAH have used the McGill pain [142729] and DNP-4 [30] questionnaire for assessing the neuropathic component of pain and none have used validated scales such as the NPSI and PDQ; however, in the present study we have used the PDQ and NPSI to assess the neuropathic component of the pain.
An upcoming area in pain research is to find the predisposing factors or vulnerability genes for chronic pain development, as it provides an opportunity to form preventive and therapeutic strategies for each patient. The present study is the very first study that identifies the alterations in mRNA expressions of the signal transduction genes PKA, PKC, and ERK in patients with CPPP following ovarian carcinoma. In a recent study by Foulkes and Wood in 2008 [31], pain genes were sub-grouped based on their functions: Genes responsible for signal transduction (P2RX3, P2RY, BDKRB1, 2, Htr3A, ACCNS, TRPV4, TRPC/P and ACCN1, 2, PKA, PKC, ERK), genes responsible for signal conduction (NR1,2; GR1A1, GR1C1-5 and NKR1, CACNA1A-5, CACNA2D1), genes responsible for synaptic transmission (Sodium and Potassium ion channels), and genes responsible for pain modulation (inflammatory factors, growth factors and cytokines acting centrally and peripherally via glialcell).
Various signal transduction molecules responsible for changes in gene regulation are Protein phosphatase 2, Calcium/Calmodulin dependent Protein kinase (PK) II α, Stress activated PK γ (JNK1) and α (JNK 2), MAPK 10 (JNK3), MAPK 1 (ERK2), MAPK 6 (ERK 3), MAPK 14 (P38), PKC α and PKCβ [9]. In the current study, the focus was on the m-RNA of the expression of the signal transduction genes, i.e. PKA, PKC, and ERK in patients with chronic persistent surgical pain. Protein kinases such as PKA, PKC, and ERK have been found to play an important role in the central sensitization of spinal cord neurons [101113]. Recently gene array studies on animals have demonstrated that peripheral nerve injury causes marked changes in the dorsal root ganglion and dorsal spinal cord [9]. Zhang et al. [9] in 2005 clearly demonstrated that expression of both PKC and ERK are up-regulated in dorsal root ganglion lesioning; however, PKC α and β1 are markedly upregulated in the neurons in lamina I-IV of the dorsal root of spinal cord of rats after peripheral axonotomy. Similarly, in the present study a significant up-regulation in the mRNA expression of signal transduction genes i.e., PKA, PKC, and ERK was observed in patients with CPPP, i.e. patients with a VAS > 4/10 on postoperative day 90. Similarly, on correlation of the ΔΔct values of various genes with the VAS score at various designated intervals in all patients labelled to have CPPP; a significant positive correlation was observed on postoperative day 90. The up-regulation of the mRNA expression of PKA, PKC, and ERK genes and its positive correlation with the VAS score in patients with CPPP show its possible association in the pathogenesis of CPPP.
The limitation of the current study is the small sample size and data from a single academic institute. In view of the fact that our institution receives only 1-2 cases/month, as per the hospital statistics, it has restricted us to only 21 cases with a minimum of 4 months follow up for each patient.
To conclude, the overall incidence of CPPP in patients undergoing staging laparotomy for ovarian carcinoma is observed to be 38.1% (8 out of 21 patients) at the end of postoperative day 90. A significant up-regulation in the mRNA expression of signal transduction genes, i.e. PKA, PKC, and ERK and a positive correlation with the VAS score was observed in the patients with CPPP. However, we suggest a prospective multi-centric trial with a larger sample size to confirm the findings of the present study.
Go to : 
References
1. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006; 367:1618–1625. PMID: 16698416.

2. Kehlet H, Edward RR, Buvanendran A. Persistent postoperative pain: pathogenic mechanism and preventive strategies. Seattle (WA): International Association for the Study of Pain/IASP Press;2012. p. 133–146.
3. Mikkelsen T, Werner MU, Lassen B, Kehlet H. Pain and sensory dysfunction 6 to 12 months after inguinal herniotomy. Anesth Analg. 2004; 99:146–151. PMID: 15281521.

4. Brandsborg B, Nikolajsen L, Kehlet H, Jensen TS. Chronic pain after hysterectomy. Acta Anaesthesiol Scand. 2008; 52:327–331. PMID: 18269384.

5. Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012; 59:B4374. PMID: 22239844.
6. Brandsborg B, Dueholm M, Nikolajsen L, Kehlet H, Jensen TS. A prospective study of risk factors for pain persisting 4 months after hysterectomy. Clin J Pain. 2009; 25:263–268. PMID: 19590472.

7. Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005; 25:7317–7323. PMID: 16093381.

8. Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004; 24:10410–10415. PMID: 15548656.

9. Zhang X, Xiao HS. Gene array analysis to determine the components of neuropathic pain signaling. Curr Opin Mol Ther. 2005; 7:532–537. PMID: 16370375.
10. Li G, Lu X, Zhang S, Zhou Q, Zhang L. mTOR and Erk1/2 signaling in the cerebrospinal fluid-contacting nucleus is involved in neuropathic pain. Neurochem Res. 2015; 40:1053–1062. PMID: 25846007.

11. Melemedjian OK, Khoutorsky A. Translational control of chronic pain. Prog Mol Biol Transl Sci. 2015; 131:185–213. PMID: 25744674.

12. Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, et al. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain. 2008; 4:26. PMID: 18631385.

13. Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol. 2005; 564:907–921. PMID: 15760935.

14. Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci Lett. 2008; 437:180–183. PMID: 18455869.

15. Brandsborg B, Nikolajsen L, Hansen CT, Kehlet H, Jensen TS. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology. 2007; 106:1003–1012. PMID: 17457133.
17. Poobalan AS, Bruce J, Smith WC, King PM, Krukowski ZH, Chambers WA. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain. 2003; 19:48–54. PMID: 12514456.

18. Wallace MS, Wallace AM, Lee J, Dobke MK. Pain after breast surgery: a survey of 282 women. Pain. 1996; 66:195–205. PMID: 8880841.

19. Gupta A, Gandhi K, Viscusi ER. Persistent postsurgical pain after abdominal surgery. Tech Reg Anesth Pain Manag. 2011; 15:140–146.

20. Obata H, Saito S, Fujita N, Fuse Y, Ishizaki K, Goto F. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anaesth. 1999; 46:1127–1132. PMID: 10608205.

21. Gottschalk A, Smith DS, Jobes DR, Kennedy SK, Lally SE, Noble VE, et al. Preemptive epidural analgesia and recovery from radical prostatectomy: a randomized controlled trial. JAMA. 1998; 279:1076–1082. PMID: 9546566.

22. Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008; 101:77–86. PMID: 18434337.

23. Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology. 2010; 112:514–515. PMID: 20124977.
24. Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, et al. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology. 2006; 104:417–425. PMID: 16508387.

25. Strulov L, Zimmer EZ, Granot M, Tamir A, Jakobi P, Lowenstein L. Pain catastrophizing, response to experimental heat stimuli, and post-cesarean section pain. J Pain. 2007; 8:273–279. PMID: 17113350.

26. Nielsen PR, Nørgaard L, Rasmussen LS, Kehlet H. Prediction of post-operative pain by an electrical pain stimulus. Acta Anaesthesiol Scand. 2007; 51:582–586. PMID: 17430320.

27. de Andrade DC, Ferreira KA, Nishimura CM, Yeng LT, Batista AF, de Sá K, et al. Psychometric validation of the Portuguese version of the Neuropathic Pain Symptoms Inventory. Health Qual Life Outcomes. 2011; 9:107. PMID: 22128801.

28. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006; 22:1911–1920. PMID: 17022849.

29. Bruce J, Poobalan AS, Smith WC, Chambers WA. Quantitative assessment of chronic postsurgical pain using the McGill Pain Questionnaire. Clin J Pain. 2004; 20:70–75. PMID: 14770045.

30. Kurita GP, Sjøgren P. Pain management in cancer survivorship. Acta Oncol. 2015; 54:629–634. PMID: 25855865.

Go to : 




 Citation
Citation Print
Print




 XML Download
XML Download