This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Nefopam has been known as an inhibitor of the reuptake of monoamines, and the noradrenergic and/or serotonergic system has been focused on as a mechanism of its analgesic action. Here we investigated the role of the spinal dopaminergic neurotransmission in the antinociceptive effect of nefopam administered intravenously or intrathecally.
Methods
The effects of intravenously and intrathecally administered nefopam were examined using the rat formalin test. Then we performed a microdialysis study to confirm the change of extracellular dopamine concentration in the spinal dorsal horn by nefopam. To determine whether the changes of dopamine level are associated with the nefopam analgesia, its mechanism was investigated pharmacologically via pretreatment with sulpiride, a dopaminergic D2 receptor antagonist.
Results
When nefopam was administered intravenously the flinching responses in phase I of the formalin test were decreased, but not those in phase II of the formalin test were decreased. Intrathecally injected nefopam reduced the flinching responses in both phases of the formalin test in a dose dependent manner. Microdialysis study revealed a significant increase of the level of dopamine in the spinal cord by intrathecally administered nefopam (about 3.8 fold the baseline value) but not by that administered intravenously. The analgesic effects of intrathecally injected nefopam were not affected by pretreatment with sulpiride, and neither were those of the intravenous nefopam.
Conclusions
Both the intravenously and intrathecally administered nefopam effectively relieved inflammatory pain in rats. Nefopam may act as an inhibitor of dopamine reuptake when delivered into the spinal cord. However, the analgesic mechanism of nefopam may not involve the dopaminergic transmission at the spinal level.
Go to :

Keywords: Analgesia, Dopamine, Microdialysis, Nefopam, Spinal cord, Sulpiride
INTRODUCTION
Nefopam is a centrally-acting, non-opiate, non-steroidal antinociceptive drug of the benzoxazocine class [
1]. It was developed in the 1960s and widely used in many countries including Korea, for the relief of moderate to severe pain [
23456]. Although the analgesic action mechanisms of nefopam are not fully understood, previous studies have suggested that the inhibition of the reuptake of monoamines in the central nervous system is involved in its effects. Nefopam was reported as an inhibitor of norepinephrine and serotonin reuptake at analgesic doses in mice [
3]. However, there are some controversies about their roles in the nefopam-produced analgesia. An alpha2-adrenoceptor antagonist RX821002 inhibited nefopam antinociception in the tail immersion test and the abdominal constriction assay [
7]. In another report, an alpha2-adrenoceptor antagonist yohimbine did not significantly alter the analgesic effect of nefopam but the 5-HT (5-hydroxytriptamine)
1B receptor antagonist GR127935, and 5-HT
2C receptor antagonist RS102221, partially reversed nefopam antinociception, in the writhing and formalin tests, respectively [
8]. In a recent study, spinal noradrenergic modulation was shown to play an important role in the nefopam analgesia against inflammatory pain in rats [
9]. Therefore the roles of noradrenergic or serotonergic components of monoaminergic transmission in the nefopam analgesia remain unclear.
Alternatively, the nefopam analgesia might also be mediated by dopaminergic transmission [
1]. However there is a paucity of data about the involvement of dopamine in the antinociceptive action of nefopam. Dopamine depletion induced by 6-hydroxydopamine and desipramine markedly reduced the nefopam analgesia measured with hot plate tests in the rats [
2]. Contrastingly, the dopaminergic D2 receptor antagonist sulpiride did not affect nefopam analgesia in the mice writhing test, but attenuated nefopam analgesic activity in the formalin test [
8]. To the authors' knowledge, the efficacy of nefopam as an inhibitor of dopamine reuptake has not been evaluated in vivo.
The purpose of the current study was to evaluate the role of dopaminergic neurotransmission in the antinociceptive effect of nefopam. We examined the analgesic effect of intrathecally or intravenously delivered nefopam using the formalin test and we performed the microdialysis study to measure the level of extracellular dopamine in the spinal dorsal horn after nefopam administration. Also, we investigated its pharmacological mechanism using a dopaminergic receptor antagonist.
Go to :

MATERIALS AND METHODS
The current study was approved by The Institutional Animal Care and Use Committee of the authors' university. Experiments were conducted using male Sprague-Dawley rats (225-250 g) housed in a temperature-controlled (22-23℃) room with an alternating 12-hour light/dark cycle. Access to water and food were allowed to access freely. For intrathecal drug administration, a polyethylene-10 (PE-10) catheter was implanted into the intrathecal space to deliver the experimental drugs as described by Yaksh and Rudy [
10]. Under inhalational anesthesia with sevoflurane, the dorsal part of the neck was dissected to expose the atlantooccipital membrane through which a PE-10 catheter was introduced and advanced caudally 8.5 cm to the level of the lumbar enlargement. The external end of the catheter was tunneled to the top of the head and plugged with a piece of wire. Any rats exhibiting motor deficits after the surgery were sacrificed immediately with an anesthetic overdose. Each rat was given a subcutaneous injection of 5 ml saline before the end of the surgery, and the animals were housed in individual cages for recovery at least of 5 days.
As a nociceptive test, the formalin test was performed by an investigator blinded to the experimental drug. Rats restrained in a cylinder were injected with 50 µl of 5% formalin solution into the plantar surface of the hindpaw using a 30 gauge needle, as described previously [
11]. The formalin injected animals exhibited a characteristic flinching response, which represents initial acute nociception (0-9 m, phase I, acute pain) by direct stimulation of the peripheral nociceptors, and a following facilitated state of spinal dorsal horn neurons as well as peripheral sensitization (10-60 m, phase II, facilitated pain) [
12]. This behavior was quantified by periodically counting the number of flinches of the injected paw. The flinches were counted for 1-min periods from 1 to 2 min, 5 to 6 m, and every 5 m thereafter, up to 60 m.
Nefopam hydrochloride (Acupan®, provided by Pharmbio Korea Co., Ltd.) and sulpiride (dopaminergic D2 receptor antagonist; Tocris Cookson Ltd., Bristol, UK) were used in this study. The vehicle for the drugs was saline. Intrathecal administration of the experimental drugs was performed using a hand-driven gear-operated syringe pump through an implanted catheter in a volume of 10 µl, followed by flushing the catheter with 10 µl of saline. Intravenous injection was performed by puncturing the tail vein using a 30 gauge needle.
On the day of the experiments, the rats were acclimatized in the formalin test chamber for 20 m, and were randomly allocated into one of the experimental groups. To examine the analgesic effect of nefopam, nefopam was injected intrathecally (3, 10, or 30 µg) or intravenously (1 mg/kg or 3 mg/kg) 10 m prior to the formalin test (n = 5 per group). Doses of the nefopam were chosen based on a pilot experiment in which the maximum dosage that did not cause side effects such as sedation or death was determined (e.g., 10 mg/kg intravenous nefopam caused the death of the rats). We performed a spinal microdialysis study to the measure the change in the extracellular level of dopamine after nefopam administration. Then, to determine whether the change of dopamine level was associated with the analgesic mechanism of nefopam, we administered sulpiride intravenously (3 mg/kg, n = 5) or intrathecally (100 µg, n = 5) 10 m before nefopam administration, and the formalin test was performed 10 m thereafter. The doses of sulpiride were determined by a pilot study as a maximum dosage that did not affect the control formalin response or cause side effects.
Microdialysis studies were performed as described previously [
13]. The rats were anesthetized with sevoflurane in 100% oxygen. The right femoral vein was cannulated for intravenous administration of lactated Ringer's solution at a rate of 1 ml/h. The rectal temperature was controlled at 37-38℃ with a heating pad placed under the abdomen. After performing thoracolumbar laminectomy and subsequent exposure of the L3 to L5 segment of the spinal cord, the dural surface was covered with mineral oil and the rat was placed in a stereotaxic holder. After opening the dura, a microdialysis probe (OD, 0.22 mm; ID, 0.20 mm; length, 1 mm; Eicom Co., Kyoto, Japan) was placed into the superficial layer of the dorsal horn by advancing at an angle of 30℃ and to a depth of 1 mm using a micromanipulator (model MM-3; Narishige, Tokyo, Japan). The probe was perfused with Ringer's solution (147.0 mmol/L NaCl, 4.0 mmol/L KCl, and 2.3 mmol/L CaCl
2) at a constant rate (1 µl/m) using a microsyringe pump (ESP-64; Eicom Co.). After 120 min of constant perfusion, two samples were consecutively collected to determine the basal dopamine concentrations in the dialysate [
13]. To measure the change of dopamine level by intrathecally injected nefopam, Ringer's Solution with nefopam (0.1 mM) was perfused, and for measuring those by systemically administered nefopam, 3 mg/kg of nefopam was injected through the femoral vein catheter. Thereafter, the 15-min fractions of perfusate were collected into an autoinjector (EAS-20; Eicom Co.). The samples (15 µl) were injected automatically into the HTEC-500 system (Eicom Co.) to analyze the dopamine concentrations by high-performance liquid chromatography (HPLC) with electrochemical detection. The chromatographic conditions were as follows. The mobile phase was comprised of 0.1 mol/L ammonium acetate buffer (pH 6.0), methanol (7:3 vol/vol) containing 0.05 mol/L sodium sulfonate, and 50 mg/L EDTA-2Na. The column was an EICOMPAC CAX (2.0 × 200.0 mm; Eicom Co.). The working electrode was glassy carbon (WE-3G, Eicom Co., flow rate 0.25 ml/min). The detector voltage and temperature were set at 0.45 V and 35.0℃, respectively. The retention time for dopamine was 7.1 min and the detection limit was 50 fg per injection (information provided by Eicom Co.).
Data are shown as means ± SEM. The time-response data of the behavior produced by formalin are presented as the number of flinches. The dose-response data are presented as a percentage of the control for each phase: % of control = Total flinching number with drug in phase 1(2) / Total flinching number of control in phase 1(2) × 100. The dose-response data were compared using one-way analysis of variance (ANOVA) with T3 Dunnett adjustment for post hoc analysis. The antagonistic effects of nefopam were analyzed using an unpaired t test. Microdialysis data were analyzed using repeated-measures ANOVA with Bonferroni adjustment for post hoc analysis. A P value < 0.05 was considered to indicate a statistically significant difference.
Go to :

RESULTS
An injection of formalin into the subcutaneous space of the paw of the vehicle (control) group evoked a biphasic pattern of flinching, with an early response lasting 5 to 10 m (phase I), and after a quiescent interval of 5 to 10 m, a subsequent late response of up to 60 m (phase II).
Figs. 1 and
2 show the time course and dose response curves of the intravenous and intrathecal nefopam administered 10 m prior to the formalin injection. In response to the intravenous injection of nefopam at 3 mg/kg the flinching count was decrease to 60% of control in phase I but not in phase II of the formalin test (
Fig. 1). When intrathecally injected, nefopam reduced the flinching responses dose-dependently in both phases of the formalin test with a maximum reduction at 30 µg being 49% and 44% of the control in phase I and phase II, respectively (
Fig. 2).
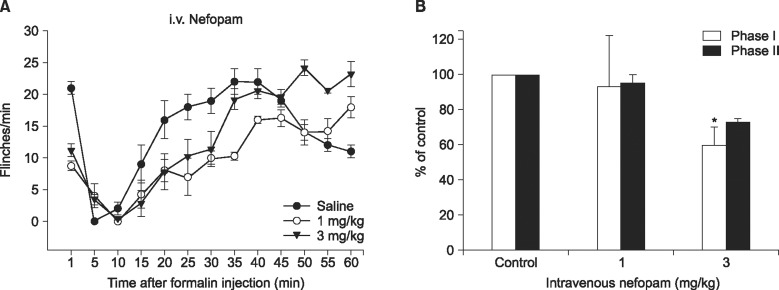 | Fig. 1Time-response (A) and dose-response data (B) of intravenously administered nefopam on flinching behavior during the formalin test. Each drug was administered 10 min before the formalin test. Data are presented as the number of flinching or the percentage of control. Intravenously administered nefopam reduced the flinching responses in phase I at a dose of 3 mg/kg. Each line or bar represents mean ± SEM of 5 rats. *P < 0.05 compared to control.
|
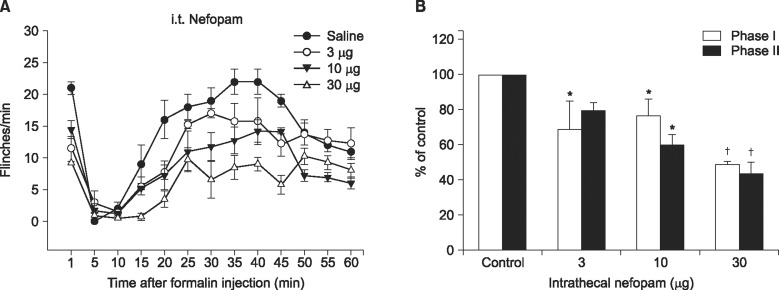 | Fig. 2Time-response (A) and dose-response data (B) of intrathecally administered nefopam on flinching behavior during the formalin test. Each drug was administered 10 min before the formalin test. Data are presented as the number of flinching or the percentage of control. Intrathecally administered nefopam reduced the flinching responses in both phases of formalin test in a dose dependent manner. Each line or bar represents mean ± SEM of 5 rats. *P < 0.05 †P < 0.001 compared to control.
|
The microdialysis study revealed a differential effect of intravenous and intrathecal nefopam on the extracellular dopamine concentration in the spinal dorsal horn. The baseline dopamine concentration in the vehicle and nefopam groups before intravenous injection was 11.8 ± 1.1 ng/µl and 9.3 ± 0.7 ng/µl, and that before intrathecal injection was 21.0 ± 4.2 ng/µl and 13.0 ± 1.8 ng/µl, respectively. A statistically significant difference was not exhibited between the groups. The extracellular concentrations of dopamine in the spinal dorsal horn did not change significantly after intravenous administration of nefopam. However, after intrathecal administration of 30 µg nefopam, the dopamine concentrations increased within 15 m after injection, peaked at 30 m to approximately 3.8 fold the baseline value, and gradually decreased thereafter. In the vehicle treated rats, the dopamine concentrations of the dialysates after injection did not show a significant change over time (
Fig. 3).
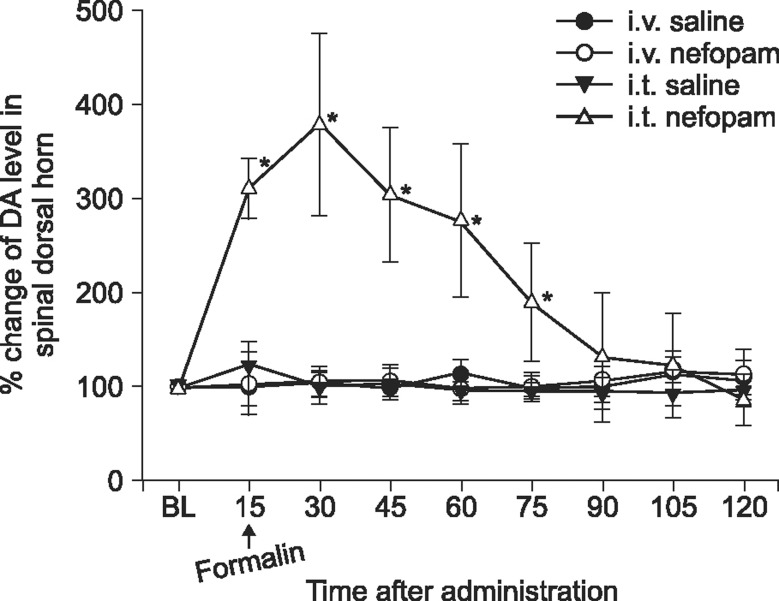 | Fig. 3Microdialysis study measuring extracellular dopamine level at the spinal dorsal horn after intravenous (3 mg/kg) or intrathecal (30 µg) delivery of nefopam. Data are presented over time as a percent change from the baseline (n = 5 in each group). *P < 0.05 compared to baseline (BL) value.
|
Nevertheless, the analgesic effects of intrathecally injected nefopam were not affected by pretreatment with sulpiride in both phases of the formalin test, and neither were those of the intravenous nefopam (
Fig. 4).
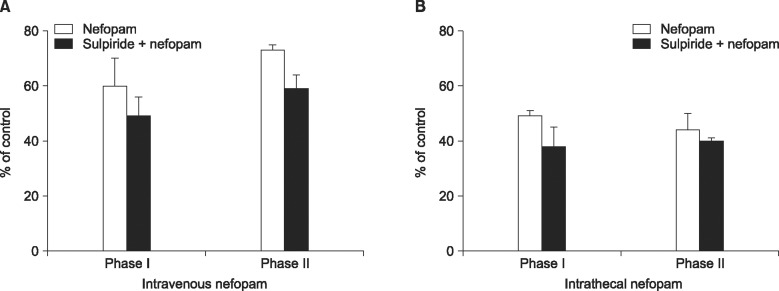 | Fig. 4The effects of intravenous pretreatment with intravenous sulpiride (3 mg/kg) on the analgesic effect of intravenous (3 mg/kg) nefopam (A), or those with intrathecal sulpiride (100 µg) on intrathecal (30 µg) nefopam (B). The antinociception produced by nefopam was not attenuated by sulpiride. Each line or bar represents mean ± SEM of 5 rats.
|
Go to :

DISCUSSION
In the current study, both intravenously and intrathecally administered nefopam reduced pain behavior induced by formalin. The microdialysis measurement of dopamine revealed that intrathecally but not intravenously delivered nefopam increases the extracellular level of dopamine in the spinal dorsal horn. Nevertheless, the blockade of the dopaminergic D2 receptor did not affect the effects of intrathecally administered nefopam. These results indicate that the analgesic mechanism of nefopam may not involve the dopaminergic transmission at the spinal level, although nefopam may act as an inhibitor of the dopamine reuptake when delivered into the spinal cord.
Nefopam has been known as an inhibitor of the reuptake of monoamines including dopamine [
13]. Nevertheless, to the authors' knowledge, this is the first study to measure the change of the extracellular level of dopamine in the spinal dorsal horn in vivo. Because nefopam was found to cross the blood-brain-barrier readily and enter the brains scanned by PET after intravenous injection of radiolabelled nefopam [
14], we assumed that both the intravenous and intrathecal nefopam would increase the dopamine level. However, only intrathecally delivered nefopam increased the level of dopamine, which could not be exhibited by the dosage of intravenous administration in the current study. Although the data are not included in the analysis, intravenous injection of nefopam at 10 mg/kg or higher doses apparently exhibited further decrease of flinching behavior in the formalin test and increase of dopamine levels in the microdialysis study, but frequently caused sedation, respiratory depression, and/or death, as documented previously. Therefore, the intravenous dose of nefopam in the current study (3 mg/kg) might have been insufficient to reach an appropriate concentration in the spinal cord to inhibit dopamine reuptake. In other words, intravenous administration of the nefopam could not increase extracellular dopamine levels at the spinal cord because of the dose-limiting side effects.
On the other hand, it is noteworthy that the dopaminergic D2 receptor antagonist did not attenuate the effect of intrathecally delivered nefopam which at the same time increased extracellular dopamine in the spinal dorsal horn, because the dopaminergic system in the spinal cord has been shown to modulate nociceptive transmission through the activation of D2 receptors [
15]. However, in numerous reports, supraspinal rather than spinal pathways have been implicated in the modulation of pain modulation through dopaminergic neurotransmission [
16171819]. Dopamine did not produce reliable antinociceptive effects when pain is evoked by phasic stimuli measuring spinal reflexes such as the tail-flick test [
17]. However, dopamine agonists produced analgesic effects when using tonic stimuli, suggesting a supraspinally mediated mechanism [
18]. Intrathecal administration of D2 receptor agonist quinpirole at a relatively large concentration exhibited only short-lived antinociceptive effects, while low systemic injection of the same agent could induce analgesic effects lasting more than 48 hr [
19]. Collectively, supraspinal rather than spinal pathways are thought to play a major role in the analgesic action of dopamine [
18]. Intrathecally delivered nefopam in a volume of 20 µl in the current study may spread no more proximally than the basal cistern and would be confined to the spinal cord [
2021]. Therefore intrathecal nefopam could not activate the supraspinal dopaminergic pathway to affect the nociception although it might have elevated the extracellular level of dopamine in the spinal cord. Consequently, we can speculate that dopaminergic modulation may not be a working mechanism for the effect of intrathecally administered nefopam. In the literature, the involvement of the dopaminergic system in the action mechanism of nefopam has been inconsistently reported. The D2 receptor antagonists haloperidol [
6] or sulpiride [
22] did not modify the antinociceptive effect of nefopam in the writhing test, which are consistent with the results of the current study. However, as described earlier, selective depletion of dopamine in the brain by 6-hydroxydopamine and desipramine attenuated nefopam's analgesic effect [
2]. In another study, sulpiride blocked nefopam analgesic effect at 3 mg/kg but not at 10 mg/kg in the mice formalin test [
8]. However, in the same study with the writhing test, sulpiride (10 mg/kg) did not modify analgesic effects produced by any doses of nefopam [
8]. The discrepancy between our data and these contrasting reports may result from methodological differences in the experiments such as the routes of drug delivery, the types and doses of drugs administered, and the types of stimuli utilized, which may determine the involvement of supraspinal and/or spinal pathways.
The analgesic action mechanisms of nefopam have been focused on the inhibition of the uptake of monoamine in the synaptic cleft [
8]. According to the result of the current study, the dopaminergic system is not thought be involved in nefopam's analgesia, at least for its applicable dose without significant side effects in rats. As described above, the noradrenergic and/or serotonergic system may be associated with nefopam's effects, although further investigations including in vivo evaluation of those transmitters' change are warranted. In addition, the glutamatergic system, transient receptor potential vanilloid subtype 1, and voltage-sensitive calcium channels have been suggested as possible pathways that underlie the nefopam analgesia [
222324].
There are some limitations to this study. First, although the analgesic effect produced by intravenous nefopam was not reversed by dopaminergic D2 receptor antagonists, the involvement of supraspinal dopaminergic pathway(s) could not be excluded. Pharmacological investigations utilizing supraspinal administration of nefopam are needed in a future study. Second, the intrathecally administered nefopam showed analgesic effects on both phases but the intravenous one exhibited an antinociceptive effect on only phase I of the formalin test; thus, the differential mechanism of the systemic and spinal action of nefopam on acute and facilitated pain states should be considered in a future study. Finally, the action mechanism of nefopam could not be determined by the current study. Thus, further studies are needed to investigate the role of those possible mechanisms described above in nefopam-induced analgesia.
In conclusion, both the intravenously and intrathecally administered nefopam effectively relieved inflammatory pain in rats. Nefopam may act as an inhibitor of dopamine reuptake when delivered into the spinal cord. However, the analgesic mechanism of nefopam may not involve the dopaminergic transmission at the spinal level.
Go to :

ACKNOWLEDGEMENTS
This study was supported by a grant from the Ministry of Science, ICT and Future Planning, and National Research Foundation of Korea (NRF-2012R1A1A1004608).
Go to :

References
1. Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain. 2014; 27:103–111. PMID:
24748937.

2. Esposito E, Romandini S, Merlo-Pich E, Mennini T, Samanin R. Evidence of the involvement of dopamine in the analgesic effect of nefopam. Eur J Pharmacol. 1986; 128:157–164. PMID:
3098570.

3. Fuller RW, Snoddy HD. Evaluation of nefopam as a monoamine uptake inhibitor in vivo in mice. Neuropharmacology. 1993; 32:995–999. PMID:
7507578.

4. Hunskaar S, Fasmer OB, Broch OJ, Hole K. Involvement of central serotonergic pathways in nefopam-induced antinociception. Eur J Pharmacol. 1987; 138:77–82. PMID:
2442003.

5. Rosland JH, Hole K. The effect of nefopam and its enantiomers on the uptake of 5-hydroxytryptamine, noradrenaline and dopamine in crude rat brain synaptosomal preparations. J Pharm Pharmacol. 1990; 42:437–438. PMID:
1979627.

6. Vonvoigtlander PF, Lewis RA, Neff GL, Triezenberg HJ. Involvement of biogenic amines with the mechanisms of novel analgesics. Prog Neuropsychopharmacol Biol Psychiatry. 1983; 7:651–656. PMID:
6141608.

7. Gray AM, Nevinson MJ, Sewell RD. The involvement of opioidergic and noradrenergic mechanisms in nefopam antinociception. Eur J Pharmacol. 1999; 365:149–157. PMID:
9988097.

8. Girard P, Coppé MC, Verniers D, Pansart Y, Gillardin JM. Role of catecholamines and serotonin receptor subtypes in nefopam-induced antinociception. Pharmacol Res. 2006; 54:195–202. PMID:
16750379.

9. Jeong SH, Heo BH, Park SH, Kim WM, Lee HG, Yoon MH, et al. Spinal noradrenergic modulation and the role of the alpha-2 receptor in the antinociceptive effect of intrathecal nefopam in the formalin test. Korean J Pain. 2014; 27:23–29. PMID:
24478897.

10. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036. PMID:
14677603.

11. Cho SY, Park AR, Yoon MH, Lee HG, Kim WM, Choi JI. Antinociceptive effect of intrathecal nefopam and interaction with morphine in formalin-induced pain of rats. Korean J Pain. 2013; 26:14–20. PMID:
23342202.

12. Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992; 12:3665–3670. PMID:
1326610.

13. Lee HG, Choi JI, Yoon MH, Obata H, Saito S, Kim WM. The antiallodynic effect of intrathecal tianeptine is exerted by increased serotonin and norepinephrine in the spinal dorsal horn. Neurosci Lett. 2014; 583:103–107. PMID:
25233863.

14. Smith DF, Glaser R, Gee A, Gjedde A. [11C]Nefopam as a potential PET tracer of serotonin reuptake sites. In : Myers R, Cunningham V, Bailey D, Jones T, editors. Quantification of brain function using PET. San Diego (CA): Academic Press;1996. p. 38–41.
15. Millan MJ. Descending control of pain. Prog Neurobiol. 2002; 66:355–474. PMID:
12034378.

16. Potvin S, Grignon S, Marchand S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse. 2009; 63:390–402. PMID:
19173266.

17. Franklin KB. Analgesia and abuse potential: an accidental association or a common substrate? Pharmacol Biochem Behav. 1998; 59:993–1002. PMID:
9586860.

18. Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999; 65:2269–2287. PMID:
10597883.

19. Cobacho N, de la Calle JL, Paíno CL. Dopaminergic modulation of neuropathic pain: analgesia in rats by a D2-type receptor agonist. Brain Res Bull. 2014; 106:62–71. PMID:
24959942.

20. Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003; 55:1007–1041. PMID:
12935942.

21. Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: a novel method for the experimental study of opioid tolerance. Anesth Analg. 2006; 103:714–720. PMID:
16931686.

22. Ohkubo Y, Nomura K, Yamaguchi I. Involvement of dopamine in the mechanism of action of FR64822, a novel non-opioid antinociceptive compound. Eur J Pharmacol. 1991; 204:121–125. PMID:
1839620.

23. Novelli A, Díaz-Trelles R, Groppetti A, Fernández-Sánchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005; 28:183–191. PMID:
15714253.

24. Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004; 1013:249–255. PMID:
15193535.

Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download