This article has been
cited by other articles in ScienceCentral.
Abstract
Background
This study was designed to assess serum vitamin D status (25-OHD) in the fibromyalgia (FM) patients and to compare it with a healthy control group. It also aimed to investigate the correlation of serum vitamin D level with FM symptom severity and invalidation experiences.
Methods
A total of 74 consecutive patients with FM and 68 healthy control participants were enrolled. The eligible FM patients completed the Illness Invalidation Inventory (3*I), the Revised Fibromyalgia Impact Questionnaire (FIQR) and a short-form health survey (SF-12). Venous blood samples were drawn from all participants to evaluate serum 25-OHD levels. Mann-Whitney tests and multiple logistic regression analyses were performed and Spearman's correlations were calculated.
Results
88.4% of FM patients had low levels of serum 25-OHD. FM patients had significantly higher level of serum 25-OHD than the control group (17.24 ± 13.50 and 9.91 ± 6.47 respectively, P = 0.0001). There were no significant correlations between serum 25-OHD levels and the clinical measures of disease impact, invalidation dimensions, and health status. Multiple logistic regression analyses revealed that an increased discounting of the disease by the patient's spouse was associated with a 4-fold increased risk for vitamin D deficiency (OR = 4.36; 95% CI, 0.95–19.87, P = 0.05).
Conclusions
This study showed that although high rates of vitamin D insufficiency or deficiency were seen among FM patients and healthy non-FM participants, but it seems there was no intrinsic association between FM and vitamin D deficiency. Addressing of invalidation experience especially by the patient's spouse is important in management of FM.
Go to :

Keywords: Chronic pain syndrome, Fibromyalgia, Invalidation, Iran, Surveys and Questionnaires, Vitamin D
INTRODUCTION
It is proven that vitamin D deficiency can causes classic musculoskeletal disease such as osteomalacia and myopathy [
1]. But how can vitamin D deficiency can leads to chronic nonspecific pain and FM is not clear. Recent case control studies and one systematic review shows that there is no difference in serum vitamin D levels between healthy persons and Fibromyalgia patients [
2345], but some other studies [
678] reported that chronic widespread pain is associated with lower levels of serumic calciferol.
Without considering the pain, vitamin D levels can affect other FM symptoms such as anxiety, depression and sleep disorders [
91011]. Recent studies demonstrate a role for calciferol deficiency in the development of symptoms of wake impairment commonly associated with sleep disorders [
11]. As already defined, the relationship between pain and psychiatric disorders, as well as sleep disorders, in FM is complex and bidirectional [
1213]. Distress and sleep disorders can be both a cause and consequence of pain. Increased pain perception associated with sleep deprivation reportedly is associated with increased IL-6, an inflammatory marker also known to be elevated in patients with low 25OHD [
1114].
On the other hand, because these symptoms have a subjective nature and they don't have physical or laboratory features in FM, patients can understand disbelief and mistrust about the rightfulness of their illness in family and/or social interactions. This situation was recently termed as 'invalidation' [
15]. In lots of these cases, comorbidities destroy the patient's quality of life significantly, and also end in unemployment and isolation from social life. So, invalidation leads to maladaptive illness behaviors, including withdrawal from social life, ceasing of pleasurable activities, and a decrease in activity and exercise [
1516]. Consequently, it can potentially decrease outdoor activity and increases risk for hypovitaminosis D.
This investigation aimed to assess serum vitamin D status in Iranian FM patients and compare it to a healthy control group. It also intended to evaluate the correlation of serum vitamin D level with FM symptoms and invalidation experience in FM patients.
Go to :

MATERIALS AND METHODS
This cross-sectional study was carried out in the rheumatology outpatient clinic of a teaching hospital in Rasht, Iran from April 2013 to September 2013. All consecutive subjects of study aged between 18–65 years old were enrolled into two groups: FM and non-FM healthy groups. For the sake of avoiding confounding factors, we selected only female individuals in both groups. Fibromyalgia was diagnosed by an expert rheumatologist using American College of Rheumatology (ACR) 1990 classification criteria or ACR 2010 preliminary criteria [
171819]. Healthy participants, however, were selected from volunteer healthy members of the FM patients' families. Of note, these healthy participants were all gender-matched and met age inclusion criteria.
Patients were excluded if they had rheumatoid arthritis or other rheumatologic diseases, severe depression, hyperparathyroidism, hyperthyroidism, osteomalacia, osteoporosis, liver or renal disease, malabsorption, calcium or vitamin D metabolic disorder, diabetes mellitus, malignancy or pregnancy, or were administered anti-depressive and anticonvulsants drugs for the last 6 weeks, calcium or vitamin D supplements during past 3 months, systemic corticosteroids, or were unable to read or write.
All participants were asked to fill out the demographic characteristics form, the Illness Invalidation Inventory (3
*I), the Revised Fibromyalgia Impact Questionnaire (FIQR) and a short-form health survey (SF-12) [
20212223]. Then, the FM patients underwent dolorimetery examination for evaluation of tender point counts.
Then, the participants were referred to the hospital laboratory to take a blood sample. A 5 ml blood sample was drawn from an anti-cubital vein from all participants in a fasting state. Erythrocyte sedimentation rate, serum phosphorus and calcium, and alkaline phosphatase were measured with routine laboratory methods in the all participants. Serum 25-hydroxyvitamin D3 (25-OHD) levels were measured by using a commercially available chemiluminescence Immunoassay (CLIA) Kit (Liaison 25OH vitamin D total assay from DiaSorin company).
According to the literature review [
1], we considered the following cut off points for 25-OHD level: equal or more than 30 ng/ml as a normal range, 20-30 ng/ml as insufficiency, equal or less than 20 ng/ml as deficiency.
Written informed consent was obtained from all the patients. This study was approved by the Local Ethics Committee.
1. Instruments
1) The Illness Invalidation Inventory (3*I)
Invalidation was measured by the Illness Invalidation Inventory (3
*I) [
21]. This inventory includes eight items (discounting [five items] and lack of understanding [three items]) assessing the extent to which people experience invalidation with regard to each of the five sources (spouse, family, medical professionals, work environment, and social services). Participants indicated on a 5-point scale (1 = never, 2 = seldom, 3 = sometimes, 4 = often, 5 = very often) how often during the past year people within each category reacted to them in the described way. A source category that did not apply (e.g., because the patient was not employed) was skipped.
2) The Revised Fibromyalgia Impact Questionnaire (FIQR)
In our study, the clinical severity and progress of FM was assessed by the validated Persian version of the FIQR [
23]. This questionnaire has 21 individual questions. All questions are based on an 11-point numeric rating scale of 0–10 with "10" denoting the 'worst'. The FIQR is divided into three linked sets of domains as in the original FIQ: (a) "function" containing 9 questions, (b) "overall impact" (2 questions with new questions being related to the overall impact of FM on individuals' function and symptom severity, and (c) "symptoms" (containing 10 questions). The summed score is divided by 3 for function (range 0 to 90), not changed for overall impact (range 0 to 20), and is divided by 2 for symptoms (range 0 to 100). The total FIQR scores would then be the sum of the three modified domain scores [
20].
3) Medical outcome survey short form 12
Quality of life was assessed by the validated Persian version of a short-form health survey (SF-12) including eight scales to assess eight dimensions: physical functioning, physical role, social role, emotional role, bodily pain, general health, vitality and, mental health. Scores range from "0 to 100" where "0" indicates the worst and "100" the best possible condition [
22].
4) Tender-point count
Tender-points were identified by applying a dolorimeter (Force DialTM FDK20, Wagner, Instrument, EFFEGI, Italy) on each of the known anatomical locations. One assessor was trained to correctly performed the dolorimetery assessment. Tenderness at any point was considered present if some involuntary reaction of the patients to pain was observed when its pressure was lower than 4 kg/cm. The number of these tender points was recorded as the tender point count.
2. Statistical analysis
Results were expressed as a mean ± standard deviation or as a number (percentage). Normality of the distribution of variables was tested by using the one sample Kolmogorov-Smirnov test. The Mann-Whitney test was used for comparison of vitamin D distribution in patients and the control group, and the Spearman's correlation coefficients were calculated to examine the association of the severity of the FM (based on the FIQR), Quality of life (based on the SF-12) and Invalidation (based on the 3*I) with a serum level of 25OHD. Multiple logistic regression (probability for stepwise with entry 0.05, removal level = 0.10, and the Hosmer and Lemeshow test goodness-of-fit with P value = 0.999) was used to obtain insight into the correlations between variables. Significance level was set at P < 0.05 and the whole statistical analysis was carried out using SPSS for Windows version 17.0; SPSS, Chicago, IL, USA).
Go to :

RESULTS
Seventy-four FM patients and 68 healthy participants were enrolled to study. The mean age of the patients with FM was 37.96 ± 9.8 and that of the non-FM control group was 32.63 ± 10.1. Demographic, clinical and laboratory data of the FM and non-FM control groups are shown in
Table 1.
Table 1
Comparison of Demographic, Clinical and Laboratory Data between FM Patients and Non-FM Healthy Controls
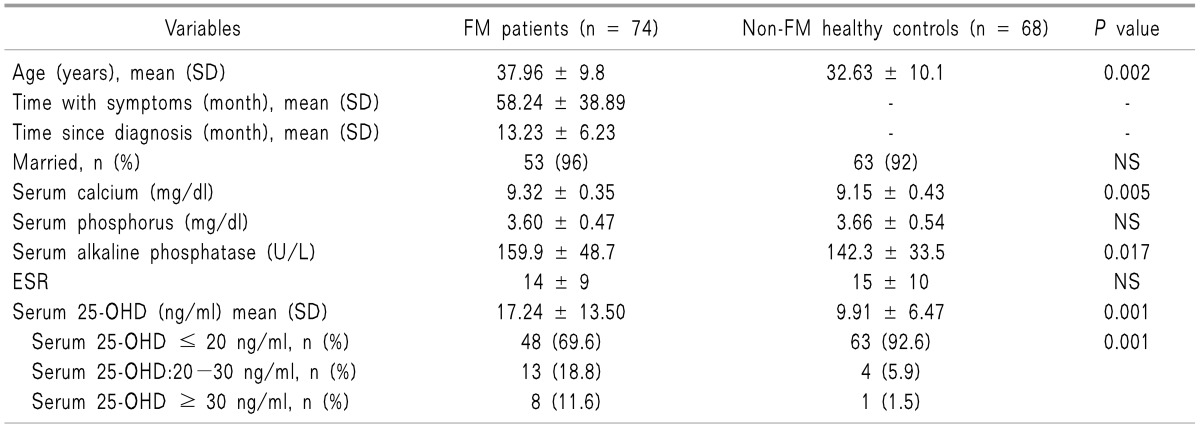

All study participants had normal serum calcium, phosphorus and alkaline phosphatase. There was no significant difference in mean serum phosphorus between the FM and non-FM control group. But, although the mean serum level of calcium and alkaline phosphatase were in normal ranges in both groups, there were significantly higher values in the FM patients than in the control group (P = 0.005 and 0.017, respectively).
FM patients had significantly higher level of serum 25-OHD than the control group (17.24 ± 13.50 and 9.91 ± 6.47 respectively,
P = 0.0001). Also, the FM group were found to have vitamin D deficiency (Serum 25-OHD ≤ 20 ng/ml) less than the control (69% and 92%, respectively,
P = 0.001). The shape of distribution and variability of serum 25-OHD and calcium levels in fibromyalgia are shown in
Fig. 1.
 | Fig. 1The shape of distribution and variability of serum 25-OHD and Ca levels in fibromyalgia patients and sex matched healthy controls.
|
FIQR total, function, overall, and symptom values in FM patients, represented as mean ± SD, were 51.8 ± 17.2, 13.5 ± 6.5, 10.6 ± 6.3, and 27.2 ± 8.0, respectively.
Discounting and lack of understanding scores for each source were reported as the average of the scale items. Scores for items 3, 5, and 8 were first reversed into the direction of lack of understanding. Discounting scores for spouse, family, medical professionals, work environment, and social services, represented as mean ± SD, were 2.00 ± 0.85, 1.96 ± 0.80, 1.66 ± 0.88, 1.82 ± 0.89, and 1.13 ± 0.18, respectively.
Similarly, lack of understanding scores for spouse, family, medical professionals, work environment and social services, represented as mean ± SD, were 1.89 ± 1.11, 1.60 ± 0.75, 2.36 ± 1.44, 2.02 ± 0.91, and 1.14 ± 0.28, respectively.
Spearman's correlation coefficients between serum 25-OHD and various parameters including RFIQ domains, invalidation sources and SF-12 subscales are shows in
Table 2.
Table 2
Spearman Correlation Coefficients between 25-OHD and Other Variables
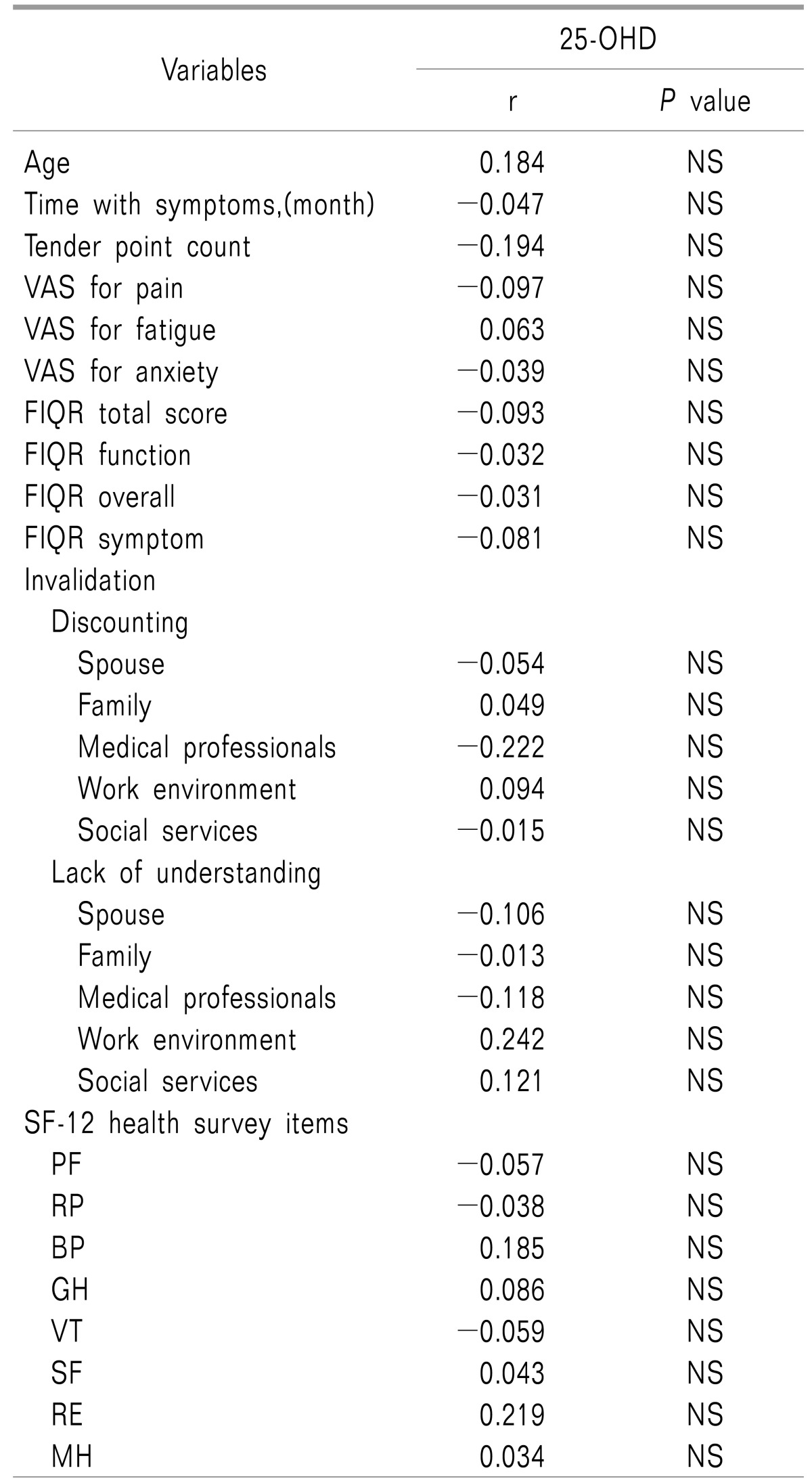

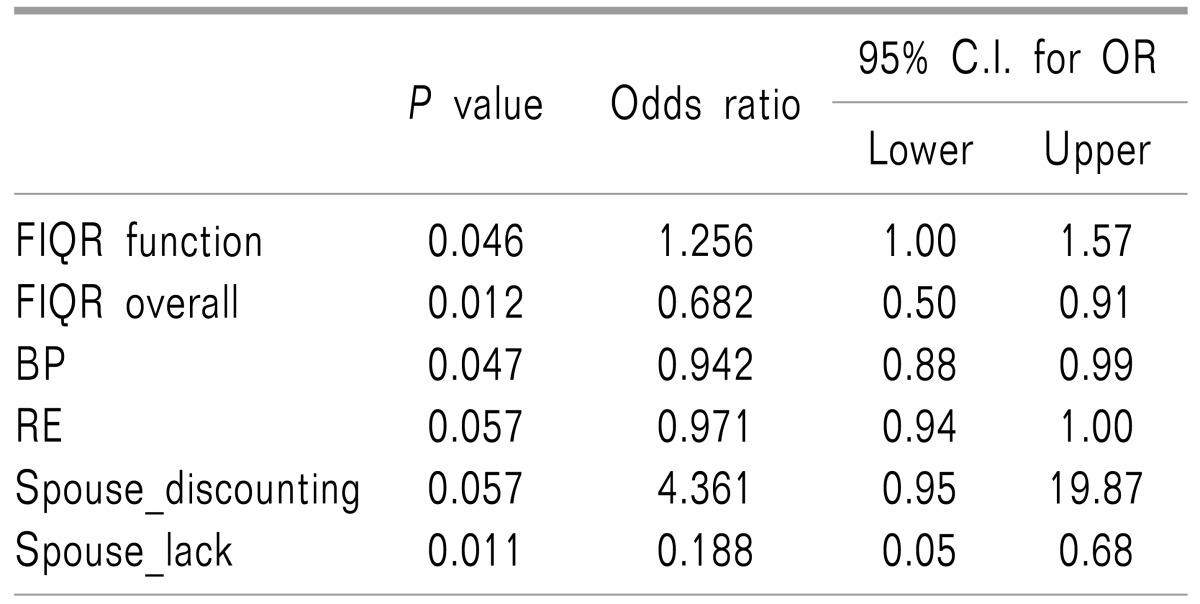
Serum 25-OHD was not correlated with the tender point count or FIQR scales for pain, fatigue and anxiety. Additionally, neither the FIQ domain's scores nor invalidation scores were correlated with serum 25-OHD. Multiple logistic regression analyses between serum 25-OHD and the FIQR domains and different sources of invalidation and SF12 subscales revealed that only increasing of discounting by the spouse was associated with a 4-fold increased risk for vitamin D deficiency (OR = 4.36; 95% CI, 0.95–19.87,
P = 0.05). Also, the odds ratio of having vitamin D deficiency was 1.25 times higher in cases with a higher RFIQ function score (OR = 1.25; 95% CI = 1.00–1.57,
P = 0.04) (
Table 3).
Table 3
Multiple Logistic Regression Analyses between 25-OHD and Other Variables

Go to :

DISCUSSION
Our results showed a high rate of vitamin D insufficiency or deficiency in FM patients and non-FM healthy participants (88.4 versus 98.5, respectively). We also showed that there was no significant association between serum 25-OHD level and clinical measures of disease impact, invalidation dimensions, and health status. But, when we analyzed the data using multiple logistic regression method, discounting by the patient's spouse was shown to be a significant predictor of calciferol deficiency in patients with FM.
Fibromyalgia is one of the most common diseases responsible for chronic musculoskeletal pain seen in the general population and outpatient rheumatology practice [
1219]. In recent years, numerous studies were designed to clear an association between a low level of calciferol and FM, two conditions that were prevalent and also lead to chronic pain. In spite of many efforts, the evidence for an association between them is unconvincing; with no progression in pain when on a vitamin D supplement [
234567824]. Many reports have showed that vitamin D deficiency can be implicated in nonspecific chronic pain or FM, and some trials have advocated a vitamin D supplement for treatment of FM [
67825], but others have not confirmed it [
23452526]. Two systematic reviews were performed, both of them concluded that the evidence base for the contribution of vitamin D deficiency to FM and using a vitamin D supplement for treatment is rather poor [
627]. de Rezende Pena et al. [
2] found no statistically significant difference between the FM and control groups with respect to mean serum concentration of 25-OHD and no correlation between vitamin D level and pain intensity. Similar results published by Warner and Arnspiger [
5] and also Straube et al. [
27] who didn't confirm the link between vitamin D deficiency and chronic pain or FM.
Our result demonstrated that a low level of 25-OHD was not seen more in FM patients, and it was not correlated with pain, or severity of FM, or poor quality of life in patients. This is in line with the idea that there is no real association between vitamin D deficiency and FM, and any observed association could be potentially explained by other mechanisms such as less sun exposure in chronic disease or associated comorbidities, such as mood disorders.
We found low levels of vitamin D in nearly all of the healthy control group, which was much higher than that seen in other studies [
4521]. Also, somewhat more surprising was the higher level of serum 25OHD in the FM group than in the healthy subjects. One possibility seems plausible. As FM patients have more pain, they are more likely to consume vitamin D supplements, which are easily accessible as the over the counter medications. Such self-treatment may be easily ignored by patients during the time of recall for this study.
Invalidation is a new definition and research on it is very scarce. This occurs in syndromes having inherent invisibility of symptoms while lacking a clear pathological basis such as in FM. It was clearly demonstrated that invalidation perceived by patients may lead to poor quality of life and FM severity in these patients [
28]. Consequently, it can potentially limit social and outdoor activity and can lead to hypovitaminosis D.
Notable is that although there was no link between low level of serum 25OHD and clinical measures of FM severity or quality of life in patients, discounting by the patient's spouse was a significant predictor of vitamin D deficiency. Discounting by their spouse had a stronger negative impact on social and physical functioning, as well as the impairment of mental health, most strongly rather than other invalidation aspects [
16]. It is the one aspect of invalidation among the various sources of its experience that seems to have a higher impact on patients [
16]. It could be expected that patients and their spouses shared daily life experiences, thoughts, and feelings, and had more empathy [
28]. We showed that for each increase in discounting by the spouse, the probability of vitamin D deficiency rose 4.3 times in FM patients. It is a striking finding which demonstrates importance of addressing of invalidation experience especially by the spouse in management of these patients.
Some limitations of our study are discussed in the following. Only female patients were evaluated, and Thus, the results of this investigation cannot be extrapolated to the opposite sex. The FM patients were a little older than the non-FM control subjects, but this doesn't seem to be clinically significant. Moreover, we didn't study our patients in a primary care setting. Therefore, they probably do not reflect the general population of fibromyalgia patients. Furthermore, our patients were not employed; So, interpretation of the results of these domains must be made with caution. It also needs to be pointed out that in our study psychiatric disorders were not studied and that they could affect invalidation and vitamin D status.
To sum up, high rates of vitamin D insufficiency or deficiency among FM subjects and non-FM healthy participants were seen, but it seems there is no intrinsic association between FM and vitamin D deficiency, so that any observed associations may be related to other factors that coexist with this disease such as lifestyle difference and mood disorders. This is the first study that tried to evaluate the correlation between serum vitamin D level and invalidation dimensions in FM patients. It is important to note and evaluate the invalidation perceptions by FM patients, especially toward their spouse, with regard to assessment and treatment of FM.
Go to :

ACKNOWLEDGEMENTS
The authors thank all the colleagues at the Guilan University of Medical Sciences, Rasht, Iran, who were coordinate in this research. The source of Funding is Guilan University of Medical Sciences.
Go to :

Notes
Go to :

References
1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–281. PMID:
17634462.

2. de Rezende Pena C, Grillo LP, das Chagas Medeiros MM. Evaluation of 25-hydroxyvitamin D serum levels in patients with fibromyalgia. J Clin Rheumatol. 2010; 16:365–369. PMID:
21085020.

3. Straube S, Derry S, Moore RA, McQuay HJ. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2010; CD007771. PMID:
20091647.

4. Tandeter H, Grynbaum M, Zuili I, Shany S, Shvartzman P. Serum 25-OH vitamin D levels in patients with fibromyalgia. Isr Med Assoc J. 2009; 11:339–342. PMID:
19697583.
5. Warner AE, Arnspiger SA. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J Clin Rheumatol. 2008; 14:12–16. PMID:
18431091.

6. McBeth J, Pye SR, O'Neill TW, Macfarlane GJ, Tajar A, Bartfai G, et al. Musculoskeletal pain is associated with very low levels of vitamin D in men: results from the European Male Ageing Study. Ann Rheum Dis. 2010; 69:1448–1452. PMID:
20498201.

7. Heidari B, Shirvani JS, Firouzjahi A, Heidari P, Hajian-Tilaki KO. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010; 13:340–346. PMID:
21199469.

8. Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003; 78:1463–1470. PMID:
14661675.

9. Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007; 26:551–554. PMID:
16850115.

10. Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010; 3:29. PMID:
21067618.

11. McCarty DE, Chesson AL Jr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014; 18:311–319. PMID:
24075129.

12. Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol. 2011; 7:518–527. PMID:
21769128.

13. Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008; 137:202–207. PMID:
18434020.

14. Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003; 89:552–572. PMID:
12720576.

15. Kool MB, van Middendorp H, Boeije HR, Geenen R. Understanding the lack of understanding: invalidation from the perspective of the patient with fibromyalgia. Arthritis Rheum. 2009; 61:1650–1656. PMID:
19950317.

16. Kool MB, van Middendorp H, Lumley MA, Bijlsma JW, Geenen R. Social support and invalidation by others contribute uniquely to the understanding of physical and mental health of patients with rheumatic diseases. J Health Psychol. 2013; 18:86–95. PMID:
22363049.

17. Bidari A, Hassanzadeh M, Ghavidel Parsa B, Kianmehr N, Kabir A, Pirhadi S, et al. Validation of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia in an Iranian population. Rheumatol Int. 2013; 33:2999–3007. PMID:
23884705.

18. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010; 62:600–610. PMID:
20461783.

19. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990; 33:160–172. PMID:
2306288.

20. Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The revised fibromyalgia impact questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009; 11:R120. PMID:
19664287.

21. Kool MB, van Middendorp H, Lumley MA, Schenk Y, Jacobs JW, Bijlsma JW, et al. Lack of understanding in fibromyalgia and rheumatoid arthritis: the Illness Invalidation Inventory (3
*I). Ann Rheum Dis. 2010; 69:1990–1995. PMID:
20498203.

22. Montazeri A, Vahdaninia M, Mousavi SJ, Omidvari S. The Iranian version of 12-item short form health survey (SF-12): factor structure, internal consistency and construct validity. BMC Public Health. 2009; 9:341. PMID:
19758427.

23. Ghavidel Parsa B, Amir Maafi A, Haghdoost A, Arabi Y, Khojamli M, Chatrnour G, et al. The validity and reliability of the persian version of the revised fibromyalgia impact questionnaire. Rheumatol Int. 2014; 34:175–180. PMID:
24381091.

24. Mateos F, Valero C, Olmos JM, Casanueva B, Castillo J, Martínez J, et al. Bone mass and vitamin D levels in women with a diagnosis of fibromyalgia. Osteoporos Int. 2014; 25:525–533. PMID:
24008400.

25. Wepner F, Scheuer R, Schuetz-Wieser B, Machacek P, Pieler-Bruha E, Cross HS, et al. Effects of vitamin D on patients with fibromyalgia syndrome: a randomized placebo-controlled trial. Pain. 2014; 155:261–268. PMID:
24438771.

26. Daniel D, Pirotta MV. Fibromyalgia--should we be testing and treating for vitamin D deficiency? Aust Fam Physician. 2011; 40:712–716. PMID:
21894281.
27. Straube S, Andrew Moore R, Derry S, McQuay HJ. Vitamin D and chronic pain. Pain. 2009; 141:10–13. PMID:
19084336.

28. Kool MB, van Middendorp H, Bijlsma JW, Geenen R. Patient and spouse appraisals of health status in rheumatoid arthritis and fibromyalgia: discrepancies and associations with invalidation. Clin Exp Rheumatol. 2011; 29:S63–S69. PMID:
22243550.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download