INTRODUCTION
Neurolytic agents, such as phenol and alcohol, have been used to ablate peripheral nerves to treat pain and spasticity [12345678910111213]. These agents were, however, not specific for neural tissue and complications have been seen involveing damage to surrounding soft tissue [41112]. These complications have included paralysis, soft tissue (muscle, vascular, skin) damage, and in the case of alcohol can cause pain on injection [41112]. Radiofrequency ablation (RFA) has become a safer alternative method for neurolysis especially for non-cancer pain. For smaller nerves such as ilioinguinal and lateral femoral cutaneous, RFA may be technically difficult given the small area of ablation and difficulty localizing these nerves.
Lidocaine has been demonstrated to be neurotoxic in multiple animal and cell culture studies [1428]. Ready et al. [18] in 1985 performed intrathecal injections of 2-32% lidocaine on rabbits and followed neurologic function for 7 days prior to harvesting the spinal cords for histology. They reported persistent neurologic deficit and major histologic changes starting at 8% concentration. Most animal studies, however, have used concentrations of 5% or less to study neurotoxicity since this the most common concentration used clinically. Previous studies used direct intra-neural injection, intrathecal injection, desheathed nerves, and cell cultures. Only one study, by Kalichman et al. [16], injected lidocaine peri-neurally by piercing the connective tissue separating the neural tissue from overlying muscle in rat sciatic nerves. They reported endoneurial edema, collapsed myelin sheaths, and axonal degeneration at 48 hours. This study, however, used a lower concentration than seen in previous studies, at 3% lidocaine.
Although animal models have shown evidence of neurotoxicity at clinically used concentrations of 5% or less, evidence of neurotoxicity in humans at these concentrations appeared to be much less than expected. This has been well documented in the use intrathecal lidocaine for spinal anesthesia and the phenomenon of transient neurologic symptoms (TNS). Schneider et al. [28] reported 4 cases of TNS using 5% lidocaine. Keld et al. [29] compared 5% lidocaine versus 0.5% bupivacaine, and found that lidocaine caused TNS in 26% of patients versus 3% with bupivacaine. Several prospective studies noted TNS incidence of 4-33% with lidocaine [3031].
Our study studied the utility of higher concentration 10% lidocaine as an alternative, neuro-specific, rapid-onset, and safer alternative to chemical neurolysis with phenol/alcohol in the canine sciatic nerve model using standard ultrasound guided peri-neural nerve block technique. The 10% concentration of lidocaine of 10% was chosen to study, since the use of neurolytic agents in clinical practice usually require injection of a contrast media to confirm correct placement and to rule out vascular uptake. The addition of these agents will reduce the local concentration of 10% lidocaine but keep the concentration above or at the 8% concentration needed for neurolysis as per the study by Ready et al. [18]. Given the previous study by Ready et al. [18], our hypothesis was that higher concentrations above the 5% that caused inconsistent TNS may cause rapid (defined in our study as within 20 minutes) histologic changes making 10% lidocaine a possible alternative agent compared to alcohol or phenol. This was a pilot study only and is limited to evaluation at one time point without direct comparison to other agents or different concentrations.
Go to : 
MATERIALS AND METHODS
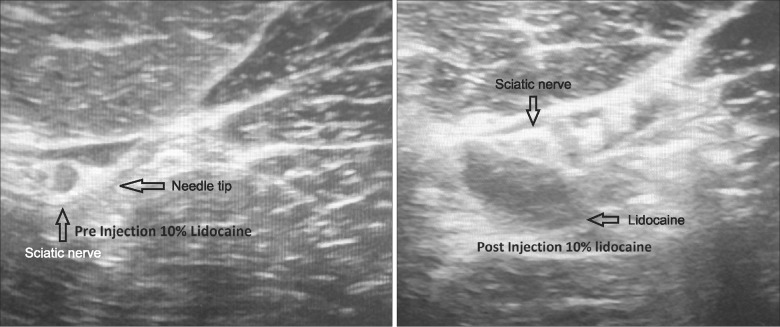
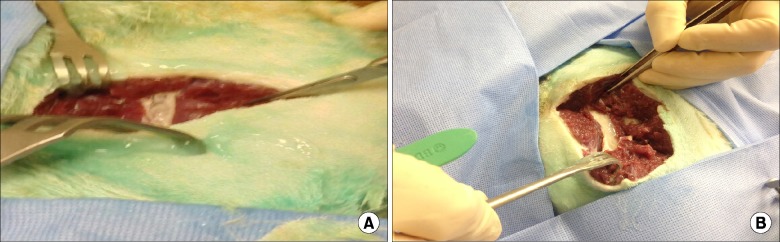
After Henry Ford Hospital (Detroit, MI) institutional review board and institutional animal care and use committee animal approval, a freshly euthanized dog (purpose bred mongrel 24 kg) was procured for the injection. The dog was placed in right lateral decubitus position and the left gluteal area was shaved and prepped with chloroprep and draped in sterile fashion (Fig. 1). Using a Venue 50 ultrasound from General Electric Healthcare (Buckinghamshire, UK), the left sciatic nerve was identified. A 22 gauge 3.5 spinal needle was guided by ultrasound adjacent to the sciatic nerve using the "in plane" technique (Fig. 2). Ten ml of 10% lidocaine preservative free compounded by Health Dimensions Compounding Pharmacy (Farmington MI) was injected under live ultrasound and demonstrated peri-neural spread (Fig. 2). After waiting 20 minutes, a scalpel was used to dissect along the spinal needle down to the sciatic nerve with gross inspection of the sciatic nerve and surrounding muscle noted (Fig. 3A). A 3 cm segment was excised and preserved in 10% buffered formalin fixative solution. The dog was placed in the right lateral decubitus position to identify the right gluteal region. The process was repeated on the right side except the sciatic nerve was injected with 10 cc 0.9% preservative free saline (Figs. 2, 3B). Both samples underwent progressive dehydration and infusion of paraffin after which they were placed on paraffin blocks. The six sections were cut at 4 µm and stained with hemoxylin and eosin. Microscopic review was performed by a pathologist from Henry Ford Hospital who was blinded to which experimental group each sample was in. The examination was performed using an Olympus BX40 microscope at 400× magnification.
Go to : 
RESULTS
The lidocaine injected nerve, on gross visualization, showed changes with loss of gross architecture on visual inspection (Fig. 3A) while the saline injected nerve did not (Fig. 3B). No gross visual changes were seen in the surrounding muscle or soft tissue seen in either saline or lidocaine injection sites (Fig. 3). On the histologic slides, the saline injected sample showed normal neural tissue (Fig. 4A). The lidocaine injected sample showed basophilic degeneration with marked cytoplasmic vacuolation in the nerve fibers with separation of individual fibers and endoneurial edema (Fig. 4B).
 | Fig. 4(A) Histology of canine sciatic nerve after peri-neural injection of 10 ml saline. 4 µm slice and stained with hemoxylin and eosin at 400× magnification: normal nerve. (B) Histology of canine sciatic nerve 20 minutes after peri-neural injection 10 ml of 10% lidocaine. 4 µm slice and stained with hemoxylin and eosin under 400× magnification. Note separation of nerve fibers, endoneurial edema, vacuolization of cytoplasm with extracellular basophilic degeneration. |
Go to : 
DISCUSSION
1. Concentration of lidocaine and neurolysis in perineural injection
The importance of a higher than clinically available concentration of lidocaine, at 10%, for neurolysis, was confirmed in our study. This higher concentration was closer to the 8% lidocaine concentration which Ready et al. [18] found to cause the most profound neurologic functional loss and histologic changes in the intrathecal rabbit animal model. Kalichman et al. [16] were one the few investigators to study the peri-neural injection of lidocaine in clinically used concentrations. Their study exposed sciatic nerves in anesthetized rats to concentration of 0.9% to 3% lidocaine. They reported that again the severity of nerve injury correlated with increased concentration. They noted that the severity of axonal loss and nerve fiber injury coincided with congestion of endoneurial vessels which our study hints at with endoneurial edema seen. The need for higher concentration of lidocaine for histologic changes and clinical changes to occur was reinforced by Kapur et al. [14]. They injected 2% lidocaine intra-neurally and peri-neurally in canine sciatic nerves, with the peri-neural injection group recovering neurologic function 3 hours post injection, while the intra-neural injection group either had longer recovery or no recovery [14]. They did not find any histologic evidence of nerve toxicity in the peri-neural group and only found damage with high pressure intra-neural injection which was avoided in our study with use of ultrasound and confirmed by dissection.
2. Comparison of lidocaine to other neurolytic agents
There has not been any direct comparison of lidocaine to alcohol for neurolysis. The only comparison with phenol and lidocaine can only be inferred from a study by Westerlund et al. [5] who compared histologic and clinical responses to glycerol, phenol, and combination of phenol/glycerol intra- and peri-neurally in rat sciatic nerves. In the study, 2% lidocaine and saline were used as controls. For the intra-neural injection group, lidocaine showed no abnormality in motor function or muscle atrophy, while the other neurolytics caused the opposite effect. Additionally trophic skin changes were noted with phenol and glycerol. For the peri-neural injection group, the same results occurred, although with less severe and shorter duration neurologic deficits. Histologic samples were obtained at 1-2 weeks. The intra-neural injection group with glycerol/phenol showed 100% endo-neural damage of the injection site while the lidocaine injected nerves showed patchy nerve fiber loss and increased number of endo-neural cells with an intact endoneurium. The peri-neural injection group with phenol showed 84-100% endo-neural damage. The lidocaine group showed no damage. As our study demonstrated, higher concentration of lidocaine was needed at 10% peri-neurally to cause significant nerve fiber histologic damage. 10% lidocaine, in our study, demonstrated endoneural damage in terms of edema and nerve fiber vacuolization similar to the Westerlunds study. It is difficult to compare the two studies, especially since our study performed histology after 20 minutes post-injection versus 1 week, as done in the previous study.
3. Extra-neural local toxicity of lidocaine
Our study did not see gross acute changes to the surrounding soft tissue after injection. Phenol and alcohol have been well known to cause significant necrosis in tissue other than neural tissue. Besides the obvious cardiac and central nervous system toxicity with lidocaine, which can be controlled by dose and image guidance, review of literature does not indicate significant soft tissue injury with lidocaine. Only two case reports or series have been published in the ophthalmology literature with cases of ptosis and diplopia after lidocaine injection for retro-orbital nerve blocks causing what was presumed to ocular muscle necrosis but not verified on histology [3233].
4. Current clinical use of high concentration lidocaine
Besides its use in spinal anesthesia, the current clinical use of high concentration lidocaine for chronic pain is anecdotal and is usually limited to commercially available concentrations of 4% and 5%. Choi and Liu [34] reported 3 cases of patients who had months of relief with peripheral nerve blocks of using 5% lidocaine with 7.5 dextrose. Their study was problematic given the small number of cases, and the concentration used by Choi is less than the 8% concentration threshold for neurolysis seen in the animal study by Ready.
The histologic results of the current investigation demonstrated that 10% lidocaine, when injected under ultrasound guidance peri-neurally around a canine sciatic nerve, did result in significant rapid histologic changes. This pilot study is limited in that only a single nerve was tested, only a single concentration was used, and results show results in a single point in time and no direct comparison was made with other clinically used agents. The authors are currently attempting to repeat this study using different concentrations over different time periods. Further animal studies and clinical studies to compare the neurolytic effects of lidocaine compared to other agents are needed.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download