This article has been
cited by other articles in ScienceCentral.
Abstract
Phantom limb pain is a phenomenon in which patients experience pain in a part of the body that no longer exists. In several treatment modalities, spinal cord stimulation (SCS) has been introduced for the management of intractable post-amputation pain. A 46-year-old male patient complained of severe ankle and foot pain, following above-the-knee amputation surgery on the right side amputation surgery three years earlier. Despite undergoing treatment with multiple modalities for pain management involving numerous oral and intravenous medications, nerve blocks, and pulsed radiofrequency (RF) treatment, the effect duration was temporary and the decreases in the patient's pain score were not acceptable. Even the use of SCS did not provide completely satisfactory pain management. However, the trial lead positioning in the cauda equina was able to stimulate the site of the severe pain, and the patient's pain score was dramatically decreased. We report a case of successful pain management with spinal cauda equina stimulation following the failure of SCS in the treatment of intractable phantom limb pain.
Go to :

Keywords: Cauda equina, Lower extremity pain, Neurostimulation, Phantom limb, Phantom limb pain, Spinal cord stimulation
Phantom limb pain is a phenomenon in which patients experience pain in a part of the body that no longer exists [
123]. Generally, phantom limb pain is considered to be neuropathic pain, and the mechanism of the symptom is assumed to be associated with an abnormality of the peripheral and/or central nervous system. Phantom limb pain can be experienced after the surgical removal of any body part such as eyes, tongue, teeth, breasts, rectum, bladder, penis, or testicles. The most common site of the pain, however, is an arm or a leg [
1].
Most management options for phantom limb pain are not effective and provide no explanation of the mechanisms to relieve the pain [
4]. A maximum efficacy of about 30% has been reported from managements such as dorsal root ganglion block, local anesthesia, sympathectomy, rhizotomy or cordotomy, spinal cord stimulation (SCS), or medications [
4]. Among the treatment modalities, spinal cord stimulation (SCS) has been utilized as a technique for the management of post-amputation pain since the early 1970s [
5]. In cases of intractable pain, SCS can be preferred [
678]. However, in the current study, we report a case of spinal cauda equina stimulation following the failure of SCS for intractable pain associated with phantom limb pain syndrome.
CASE REPORT
A 46-year-old male patient complained of severe ankle and foot pain, although he had undergone right above-the-knee (AK) amputation surgery due to severe infection three years earlier. The patient's initial visual analogue scale (VAS) score in the pain clinic was 9/10 (0 is no pain, 10 is the most severe pain imaginable). The principal site of his pain was the right foot and posterior heel area. The patient's underlying diseases included diabetes mellitus, hypertension, and variant angina. Six years earlier, he had undergone a percutaneous coronary artery intervention procedure in the left anterior descending coronary artery due to an attack of angina. Five years earlier, he had undergone off-pump coronary artery bypass surgery.
Immediately after the right AK amputation, the patient reported that he suffered from phantom limb pain. Two years earlier, the patient was referred to the pain clinic for treatment of his severe phantom limb pain. Initially, he was treated with medications such as tramadol with acetaminophen, and gabapentin 900 mg/day. The doses of tramadol with acetaminophen and gabapentin were slowly increased. Because of his severe pain, other medications such as duloxetine, amitriptyline, oxycodone with naloxone, baclofen, and other anticonvulsants such as oxcarbazepine or lamotrigine were added. The patient's final oral medication regimen was comprised of gabapentin 3600 mg/day, duloxetine 60 mg/day, amitriptyline 30 mg/day, hydromorphone 16 mg/day, tramadol 300 mg/day, lamotrigine 300 mg/day, and baclofen 30 mg/day. He had undergone an interlaminar lumbar epidural block, psoas compartment block, transforaminal epidural block, lumbar sympathetic ganglion block, pulsed radiofrequency (RF) treatment, lidocaine infusion therapy, and ketamine infusion therapy. Despite these procedures, the effect obtained was transient, lasting only a few days, and the patient's VAS score was over 7/10. Finally, we recommended a trial of SCS.
For the trial of SCS, the patient was placed on the operating table in the prone position. His back was prepared and draped aseptically. A Tuohy needle was inserted through the L2-3 interlaminar space to enter the epidural space under C-arm fluoroscopic guidance. The tip of the lead was placed at the upper T12 vertebral body (
Fig. 1) and the patient's right lower leg and ankle were stimulated. We tested the spinal stimulation and changed the parameters of stimulation for one week. Although the patient's pain was decreased from VAS 7-8/10 to VAS 5-6/10, the most severe pain site of the posterior heel could not be stimulated. After one week of lead insertion, we changed the lead position from T10 to L1 and the parameters of stimulation in the operating room. Nevertheless, the right posterior heel still could not be stimulated. In the end, the lead was removed and finally repositioned at another level, specifically the cauda equina. The dermatomes related to the patient's pain were thought to be L5, S1, and S2. A Tuohy needle was inserted through the L5-S1 interlaminar space and the tip of the lead was placed at the upper L4 vertebral body (
Fig. 2). After the stimulation of the cauda equina at the L4 and L5 levels, the severe pain site of the heel was stimulated and the patient felt the stimulation almost at his pain site (posterior ankle, heel, and foot). During the initial trial week, the patient's pain was decreased to VAS 2-3/10 and he was very satisfied with the stimulation. After one week, we performed a permanent implantation of a generator of spinal stimulation in the right subchondral area. The stimulation parameters programmed in this patient were the following: electrode, 1(+), 2(-), 3(-); amplitude 2.3 V; pulse width, 450 ms; rate, 40 Hz.
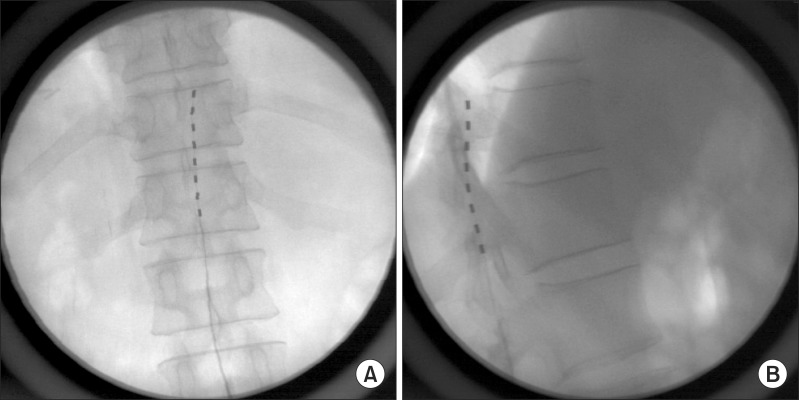 | Fig. 1(A) Fluoroscopic images of anteroposterior view. The tip of lead is placed at upper T12 vertebral body. (B) Fluoroscopic images of lateral view. The tip of lead is placed at upper T12 vertebral body.
|
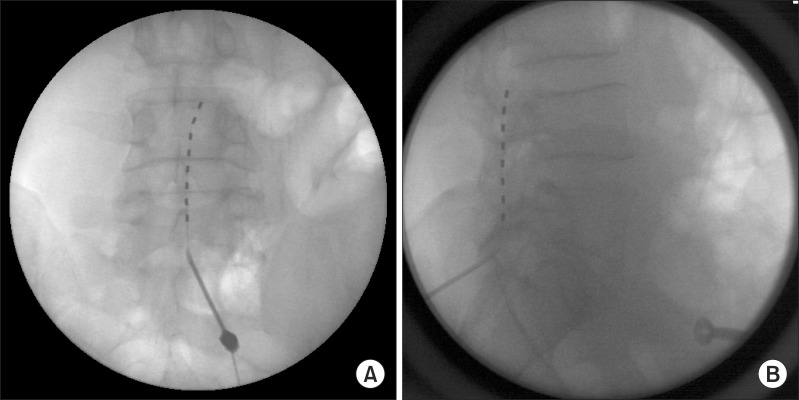 | Fig. 2(A) Fluoroscopic images of anteroposterior view demonstrating the final lead position. The tip of lead is placed at upper L4 vertebral body. (B) Fluoroscopic images of lateral view demonstrating the final lead position. The tip of lead is placed at upper L4 vertebral body.
|
During the five months of follow-up after the operation, the patient's pain score was maintained at VAS 2-3/10 and some of his medications were decreased, such as gabapentin to 2400 mg/day and tramadol to 200 mg/day. When his pain was increased (VAS 4-5/10), he received intermittent epidural blocks.
Go to :

DISCUSSION
In this case, despite several changes of the lead position, the most severe pain site – the posterior heel – was not stimulated and the decrease in the patient's pain score following SCS was not satisfactory. However, the trial lead positioning in the cauda equina was able to stimulate the severe pain site, and the patient's pain score was dramatically decreased. We were unable to find any literature regarding cauda equina stimulation for the control of intractable pain. Therefore, this case is the first report of cauda equina stimulation for pain management.
Pain arising from amputated limbs may vary greatly due to a diversity of etiologies, and that might result in diverse responses to SCS [
3]. A large survey reported that almost 80% of all amputees develop phantom limb pain, defined as pain experienced in a nonexistent part of a limb that was amputated in an operation; 68% develop pain in the remaining stump; and 62% develop low back pain associated with phantom limb pain syndrome [
3]. In an epidemiological study involving 124 upper limb amputation patients, the prevalence of phantom limb pain was 51%, that of stump pain was 49%, and that of phantom sensations was 76%; 48% of the patients experienced phantom pain a few times per day or more, and 64% experienced moderate to severe suffering from the phantom limb pain [
9]. Houghton et al. [
10] reported in a study of lower limb amputees that phantom sensations were experienced by 82% and phantom limb pain by 78%. The intensity and frequency of phantom limb pain usually diminish with time; however they do not diminish in all cases [
11]. Phantom limb pain was found to be similarly present in traumatic and vascular amputees, and was connected to the degree of preoperative pain [
10].
Phantom limb pain, the disease entity, has a variety of multifarious pathophysiologies that may comprise, overlap, and affect each other to different degrees in various individuals. In the past, it was recognized as a psychosis, but recently it has been assumed to involve changes in the peripheral nervous system and central nervous system containing the cerebral cortex [
1213]. It is assumed that psychological factors influence the disease progress and the degree of pain. The proposed pathophysiologic mechanisms are the following. First, the severed peripheral nociceptive nerve terminal may become sensitive to mechanical stimuli and so confusedly signal pain from non-painful stimuli. Second, the degeneration of nociceptive neurons may lead to inappropriate compensatory sprouting of mechanosensitive proximal endings onto the deafferented nociceptive spinal neurons. Third, the continuous sensitization of these second-order spinothalamic neurons may cause pain-level reactions to non-painful stimuli. Fourth, the somatosensory and motor cortical reorganization shifting representation adjacent to regions subserving the amputated body part may promote a dysregulated painful recognition as well as result in phantom limb sensation [
1213]. For these reasons, it is difficult to treat phantom limb pain.
In our case, the patient had been suffering from phantom limb pain in the right foot and ankle area. The most painful site was the posterior heel. Although the lead position was changed from T10 to L1 related to the expected spinal cord level for ankle and foot stimulation, the stimulation of the patient was not achieved at the major pain site. We thought that the impossibility of stimulation at the patient's ankle and foot was related to spinal cord reorganization. Ramachandran et al. reported that cortical reorganization may be one of the etiologies of phantom limb pain. This was confirmed through functional magnetic resonance imaging (fMRI) [
1415]. Flor et al. [
16] postulated that hyperexcitability at the spinal cord level was one of the pathophysiological factors involved in phantom limb pain. This hyperexcitability involves downregulation of opioid receptors, loss of inhibitory interneurons, and reorganization of spinal cord dermatomes [
16]. Eldabe et al. [
17] reported on dorsal root ganglion (DRG) stimulation in phantom limb pain patients. They stated that in spite of the poor somatotopic specificity in phantom limb pain patients, DRG stimulation may be an effective tool in the management of phantom limb pain. The advantages of DRG stimulation were the lack of paresthesia change with position, and distinct and consistent paresthesia coverage in areas of the anatomy that are hard to cover with conventional dorsal column stimulation [
17]. However, DRG stimulation requires multiple leads for coverage of multiple DRGs related to the patient's pain site. DRG stimulation also requires multiple insertions of a Tuohy needle to place each lead at each DRG. In this case, we supposed that if the L5, S1, and S2 nerves could be stimulated, the sites of the patient's pain could almost be covered. Therefore, we tried placing the electrode in the cauda equina related to the L5, S1, and S2 nerves. In this case of cauda equina stimulation, only one lead was placed for stimulation of the L5, S1, and S2 nerves.
Neuromodulation methods with different approaches and different sites have been utilized for treatment of patient pain and disability. Not only SCS but also peripheral nerve stimulation such as pudendal nerve stimulation [
18] and occipital nerve stimulation [
19], peripheral nerve field stimulation via subcutaneous leads [
20], DRG stimulation [
17], and sacral nerve stimulation [
21] have been used. Nerve stimulation has been applied to the spinal cord, DRG, spinal nerves, and peripheral nerves, among others. Therefore, the cauda equina can be the potential site of nerve stimulation. The cauda equina is transected and relatively mobile, therefore it was thought that stimulation of the cauda equina may have limitations in that it is difficult to find and to decide upon the exact stimulation site, as well as to stimulate the site continuously and consistently [
2223]. However, the spinal cord, which is the most commonly used site of stimulation, also shifts according to the patient's position changes, and the site and intensity of SCS can be changed by the patient's position. At the lower lumbar level, the position of cauda equina elements such as L4, L5, and sacral nerves are relatively definite (
Fig. 3). Takiguchi et al. [
24] reported the migration of the spinal cord and cauda equina from the T11-12 to the S1-2 disc level during position change in a study utilizing MRI. The maximum migration was observed at the L1-2 disc level, and the lower the cauda equina was located, the less the cauda equina moved during position change. So the lower level of the cauda equina, such as the lower lumbar nerves and sacral nerves, are less mobile, which can make them a suitable target for spinal stimulation. In this case, the patient reported that he almost did not feel the difference in the change of the site and intensity of the stimulation by changing position. The little change of stimulation by position change may be associated with the low mobility of the cauda equina at the lower lumbar and sacral levels, as shown in the investigation of Takiguchi et al. [
24] Although the spinal cord moves according to the patient's position, it is the most commonly used site of neurostimulation. Therefore, some mobility of the cauda equina, especially in the lower lumbar and sacral levels, may not be a contraindication to stimulation. The dermatomes of the lower lumbar and sacral nerves are related to the ankle and foot. This means that stimulation of the lower level of the cauda equina may be a possible target of stimulation for ankle and foot pain due to its lower mobility. Many amputees report a feeling of telescoping, such as the retraction of the phantom toward the remaining limb, and some patients feel that their foot and/or ankle is attached at their stump [
1]. For the treatment of phantom limb pain of the lower limb, the stimulation may be limited in the foot and ankle area. Therefore, lower lumbar cauda equina (L5, S1, and S2) stimulation can be an option for the management of phantom limb pain related to lower limb amputation. To prove the effectiveness of cauda equina stimulation, further investigations will be needed.
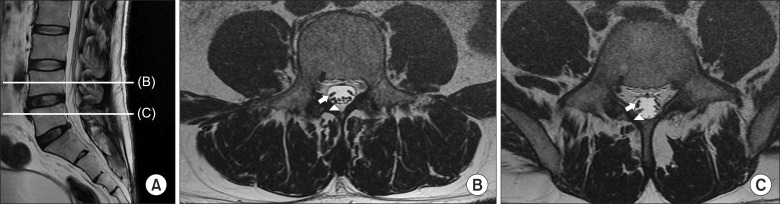 | Fig. 3(A) The patient's MRI images. Sagittal view of lumbar spine. (B) Transverse section at mid L4 vertebral body level, black arrow: Right L4 nerve root, white arrow: cauda equina of right L5, white arrow head: cauda equine of right sacral nerves. (C) Transverse section at mid L5 vertebral body level, black arrow: Right L5 nerve root, white arrow: cauda equina of right S1, white arrow head: cauda equine of right sacral nerves except S1.
|
In this case, cauda equina stimulation was effective for the management of intractable phantom limb pain and changes in the intensity or site of stimulation were minimal with the patient's position change. Although further investigation of cauda equina stimulation will be needed, cauda equina stimulation may be one of the treatment options for patients with intractable phantom limb pain.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download