MATERIALS AND METHODS
This study was carried over a period of two years. After gaining the approval of our institutional ethics committee and obtaining written informed consent from the patients and/or their healthcare providers, 79 patients of both sexes were randomly allocated into two groups. Any unexpected risks arising during the course of the study were explained to the participants and to the ethics committee in a timely manner, and proper measures were taken to overcome or minimize these risks.
The groups were as follows:
Group I, for whom a celiac plexus block was used with a bilateral needle retrocrural technique, and Group II, for whom a splanchnic nerve block with a bilateral needle technique was used.
Patients who had inoperable upper GIT tumors, including cancer of the lower third of the esophagus, stomach cancer, pancreatic cancer, and cancer of the biliary tract, with severe uncontrolled visceral pain (visual analogue scale ≥ 70/100) and who were taking the maximum tolerable dose of opioids (the dose which achieved an acceptable analgesic effect for patients with side effects tolerable for them) were included in the study.
Patients who had clotting abnormalities, local infections, uncontrolled hypotension, cardiac disorders, documented metastatic lesions, psychiatric illness affecting cooperation, a previous neurolytic block or those patients who could not tolerate a dose escalation needed to attain an analgesic effect were excluded from the study.
Also, patients were excluded from the study at any stage if they showed any other type of pain, such as somatic pain (localized, superficial sharp pain accentuated by touching the intercostal spaces) or neuropathic pain.
The complete blood picture, prothrombin time, international normalized ratio, bleeding time and clotting time were evaluated before the block performance. A recent CT scan was necessary to assess the anatomical structures at the entry site for the celiac plexus and the splanchnic nerves.
Before the block performance, all patients had an intravenous cannula (18 gauge) inserted into a large vein and securely anchored. A 500 ml solution of a lactated ringer was started. Sedation had been used to relax the patient in the form of intravenous midazolam in a dose up to 5 mg, especially at the time of alcohol injection. Then, patients were randomized into two groups: Group I, for whom a celiac plexus block was used, and Group II, for whom a splanchnic nerve block was used.
For the patients who received a celiac plexus block, each patient was placed prone on a table with a pillow under the abdomen to flex the thoraco-lumbar spine. After sterilization, the body of the first lumber vertebra was identified in the posteroanterior view of fluoroscopy, maintaining a mark on the space between T12-L1. The C-arm was moved in a caudocephalic direction to achieve alignment of the vertebral body of L1. The C-arm was then moved to an oblique position ipsilateral (20-30 degree), guided by the entrance of a transverse process of L1 in the vertebral body to achieve tunnel vision during the needle entry process. Skin infiltration was made at this point; the tip of the needle was advanced to stop at the antero-lateral border of the vertebral body of L1 (posterior to aorta) on the left side. The C-arm was then turned in the posteroanterior position to ensure contact of the needle with the vertebral body. After negative aspiration for blood or cerebrospinal fluid, the procedure was repeated for the contralateral side. While in the right side, the tip of the needle was advanced 1 to 2 cm further than the left side needle position. Five milliliter of contrast material was injected in each side for confirmation (
Fig. 1A and B). Then, 3 ml of a local anesthetic was injected, and for neurolysis, 20 ml of 70% alcohol was injected after 5 minutes to allow the local anesthetic to take action. 1 ml of 0.9% of normal saline was then injected during the withdrawal of the needle to avoid track formation.
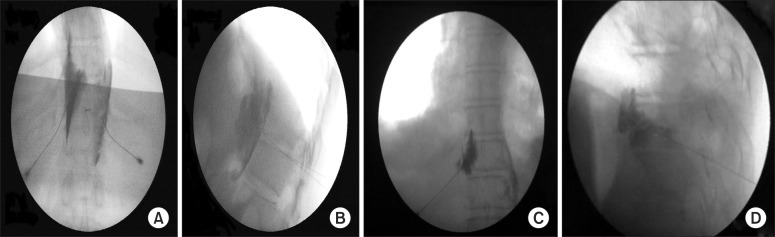 | Fig. 1(A) Celiac plexus block: posteroanterior view of both needles with the needle tip at the level of the intradiscal space between the T12 and L1 vertebrae at the facetal line. (B) Celiac plexus block: lateral view with both needles with the needle tips at the antero-lateral border of the vertebral body and dye distribution. (C) Splanchnic nerve block: posteroanterior view with the contrast material spread adherent to the T11 vertebral body (left side). (D) Splanchnic nerve block: lateral view, with the needle tip stopping at the junction of the anterior one third and posterior two thirds of the vertebral body with contrast material spread at the level of T11. 
|
For patients who had a splanchnic nerve block, the procedure was done after proper placement of the patient and sterilization. The anatomical landmarks as determined under a posteroanterior view of the C-arm included the twelfth rib and the vertebral bodies of T12 and T11.
The C-arm was rotated from approximately 15° to nearly 45° to view the edge of the vertebral body and the diaphragm. The point of entry was at the junction of the rib and the vertebral body (T11, the entry point must not exceed 4 cm from midline to avoid pleural puncture). After local anesthetic infiltration, a Chiba needle was advanced until the junction between the anterior one third and posterior two thirds of the lateral wall of the vertebral body in the lateral view of the C-arm. The C-arm was then positioned in the posteroanterior view again to ensure contact of the needle with the vertebral body. After negative aspiration for blood or cerebrospinal fluid, 3 ml of contrast material was injected. From the posteroanterior view, the contrast material will spread adhering to the T10 and/or T11 vertebral body, (
Fig. 1C and D). Then, 2 ml of local anesthetic was injected. For neurolysis, 10 ml of 70% alcohol was injected after 5 minutes to provide time for the action of the local anesthetic. An injection of 1 ml of 0.9% normal saline was then given during needle withdrawal to avoid track formation. The same procedure was repeated on the contralateral side.
Vital signs (pulse, blood pressure and oxygen saturation) had been assessed hourly for 8 hours after the procedures. Plain chest x-rays were taken of each patient 24 hours post-intervention. Incidences of side effects or complications related to the celiac or splanchnic blockade were recorded, including diarrhea, hypotension, back pain, pneumothorax, shoulder pain, and neurological affections.
Visual analogue scale assessments (0-100, where 0 means no pain while 100 indicates the maximum level of intolerable pain) were done pre-interventional, immediate post-interventional, and daily for 1 week and then every 2 weeks for 6 months. Type of pain was reevaluated during each assessment time. For simplification of the statistical analysis, the measurement on the third day was included in the result and was statistically analyzed. Neuropathic pain was diagnosed by the Douleur Neuropathique EN 4 questions (DN4) [
6]. Somatic pain was described as localized, superficial sharp pain and can often be reproduced by touching the intercostal spaces.
Opioid consumption during the follow-up period and opioids side effects, including loss of appetite, nausea or vomiting, insomnia, constipation, urinary retention, and pruritus were reported. Incidences of side effects as informed by the patient and if they required medication and changes in the type of opioid or dosage used are reported in the results.
Quality of life was assessed using the QLQ-C30 questionnaire [
7], as suggested by the European Organization for Research and Treatment of Cancer; it is composed of both multi-item scales and single-item measures. It is classified into five functional scales (role, physical, cognitive, emotional and social), three symptom scales (fatigue, pain, nausea and vomiting), and two questions assessing overall QOL. All parameters range in score from 0-100. High scores on the functional and global health scales indicate an improvement in the quality of life, whereas a higher score on the symptom scale indicates a low quality of life. Survival was reported to identify any detrimental effect of pain management protocols on patients' survival.
After the procedure, all patients were managed according to the World Health Organization analgesic ladder, including non-opioid analgesics and weak opioids (e.g., tramal 50 mg or tramadol 100 mg SR with 400 mg as a maximum daily dose). When tramadol failed to relieve pain, we shifted to strong opioids such as morphine sulphate (MST® 30 mg), hydromorphone tablets (Jurnista® 8, 16, or 32 mg) or transdermal fentanyl patches (Duragesic patches® 25, 50, 75, or 100 µg), which were administrated according patients' needs and tolerability levels. For patients who had used strong opioids other than morphine, we calculated the equivalent doses of oral morphine (the hydromorphone daily dose was multiplied by 4, while the transdermal fentanyl daily dose was multiplied by 100 to the calculate equianalgesic daily dose of oral morphine) [
8,
9] to facilitate the statistical analysis.
Statistical presentation and analysis in the present study were conducted using the mean, standard deviation, ANOVA tests, student's paired-T-tests, non-parametric tests and chi-square tests using SPSS V.21. Non-parametric tests (the Kruskal Wallis test) were used when the data were not normally distributed. Mann-Whitney tests were used to find which means were significantly different than others. The level of significance was P < 0.05. All analyses were performed using an intention-to-treat approach based on 30 patients in each group by compensating for missing data due to patients' deaths in the calculations using the last reported data value.
Go to :

RESULTS
Of one hundred and sixty seven patients assessed for eligibility, 62 patients did not have the proper inclusion criteria, and 26 patients declined to participate. Seventy nine patients were randomized during the study period (randomization was continued during the study to replace the excluded patients during the follow-up period due to changes in the characteristics of the pain). There were 38 patients in Group I and 41 in Group II, and 19 patients were excluded after randomization owing to changes in the characteristics of the pain (the development of neuropathic and/or somatic pain). Therefore, 30 patients were included in the study in each group, as shown in
Fig. 2.
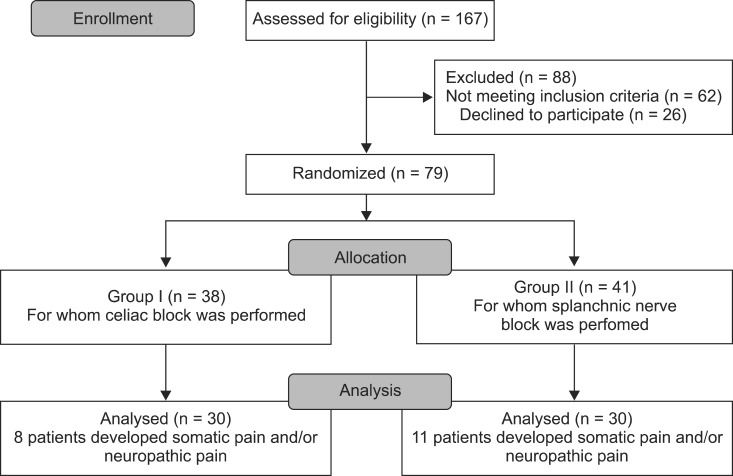 | Fig. 2Flow diagram of patient progress through the phases of the randomized trial. 
|
At enrollment, the treatment groups had comparable demographic data in terms of age, gender, body weight, and height. The time elapsed since diagnosis and the duration of pain were comparable in both groups, and the number of the patients who received radiation therapy and/or chemotherapy and the sites of the tumors in the patients included in the study were equally distributed in both groups, as shown in
Table 1.
Table 1
Patients Characteristics in Both Groups

Pain scores were comparable in both groups upon the initial assessment visit (
P = 0.75). There was a significant decrease in VAS (the visual analogue scale) in Group II versus Group I on the third day of assessment, (
P = 0.001). Meanwhile, there were no statistically differences between the groups after the first week onwards (with
P values of 0.18, 0.64, 0.21, 0.77, 0.13, 0.18, 0.20, 0.33, 0.26, 0.10, 0.11, 0.19, and 0.58, in each assessment time respectively) as shown in
Table 2. In contrast, the visual analogue scale decreased significantly in both groups in comparison with its value before the block (
P = 0.001), as shown in
Table 2.
Table 2
Comparison of VAS between Group I Versus Group II. Data are Presented as Means ± Standard Deviations and Number of Living Patients (n)
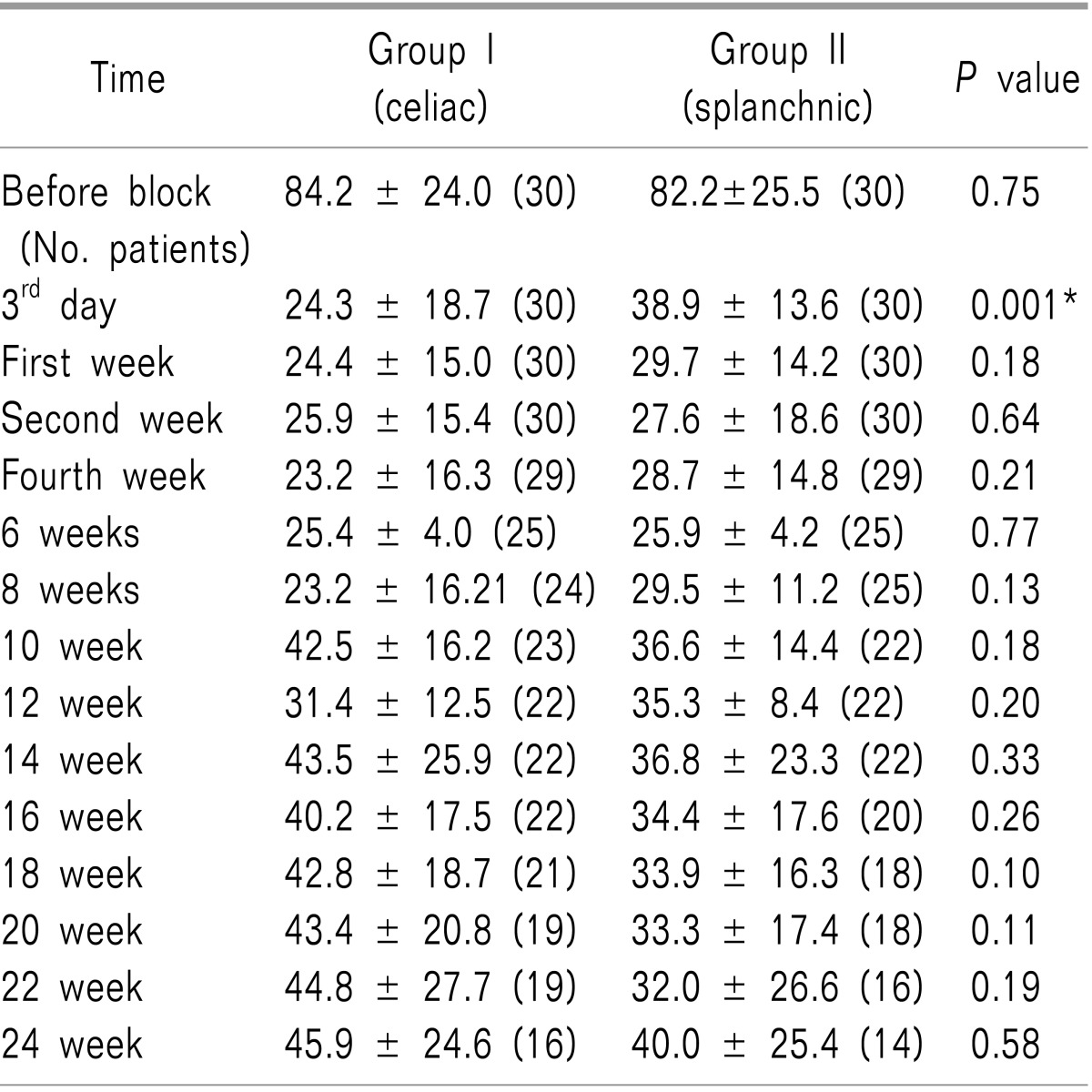

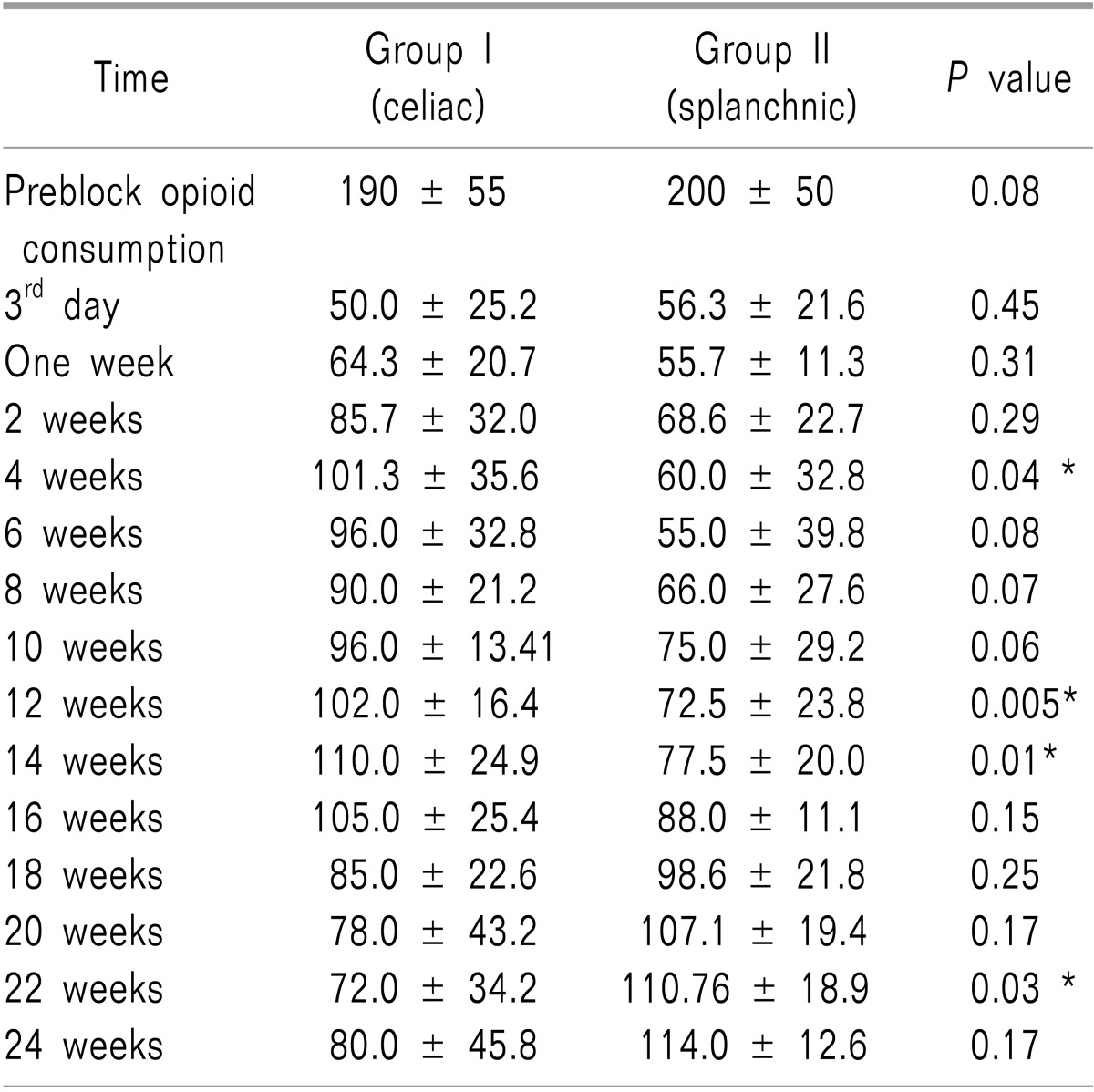
Table 3 revealed that strong opioid consumption significantly increased in Group I versus Group II at the following times of assessment (4, 12, and 14 weeks, with
P values of 0.04, 0.005, 0.01, respectively). In contrast, this rate was significantly higher in Group II versus Group I at 22 weeks (
P = 0.03).
Table 3
Strong Opioid Consumption in Equivalent Doses of MST (mg/day) during Follow-up Periods for Both Groups. Data are Presented as the Means ± Standard Deviations

Oral tramadol consumption increased significantly in Group II versus Group I at the following assessment times (20, 22, 24 weeks, with P values of 0.03, 0.001, 0.002, respectively). However, the consumption rate was significantly higher in Group I versus Group II at the following assessment times (2, 4, 6 and 8 weeks, with P values of 0.007, 0.001, 0.001 and 0.02, respectively).
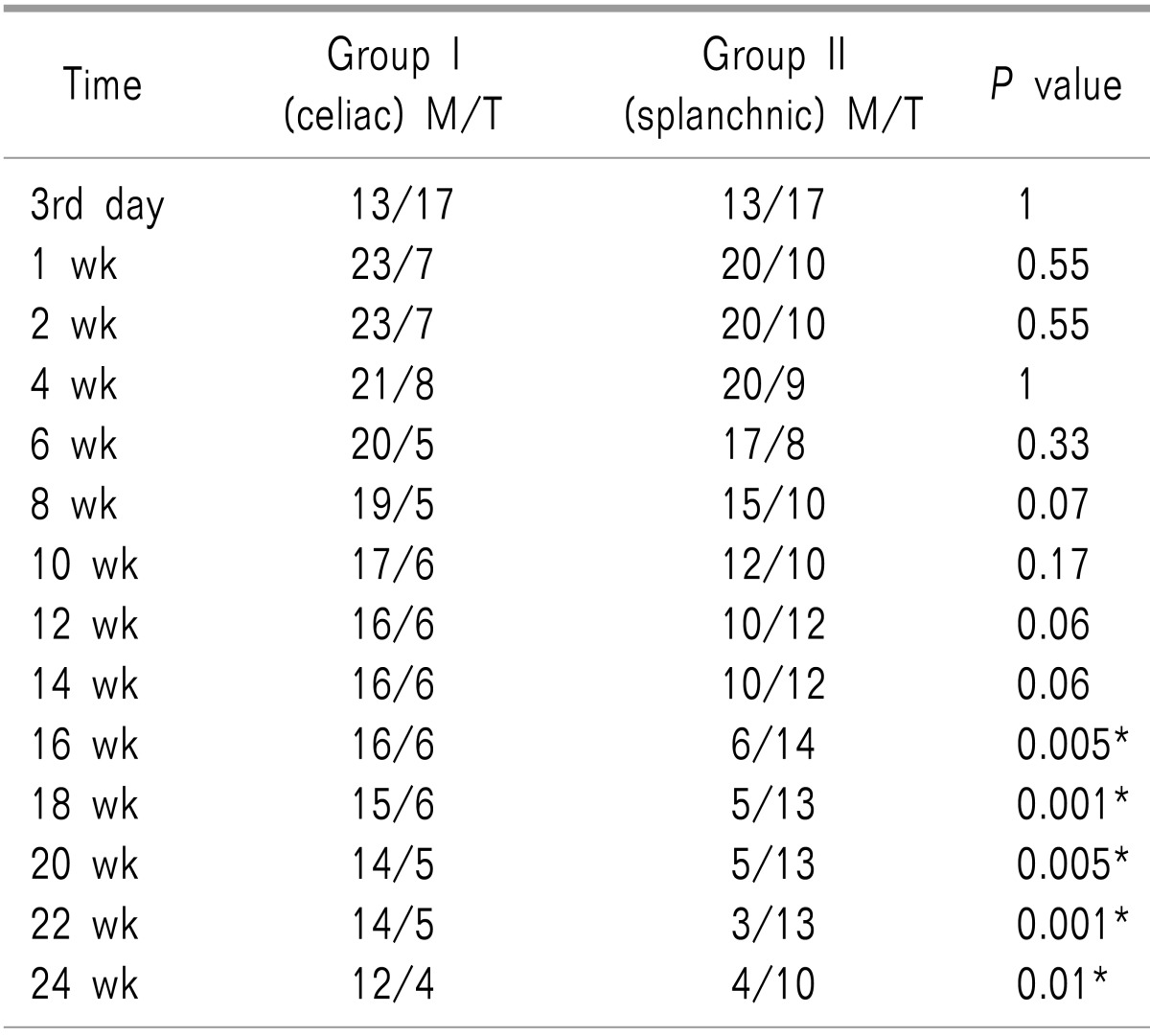
Table 4 reveals that significantly more patients retained good analgesia on tramadol in Group II from 16 weeks onwards (
P values = 0.005, 0.001, 0.005, 0.001, 0.01, in each assessment time respectively).
Table 4
Number of Patients Using Strong Opioids/Tramadol in Both Groups

Blocks were successfully performed in all patients. Self-limiting diarrhea was the most common complication, occurring in 20 patients (who underwent celiac and splanchnic nerve blocks); this was resolved within 2-5 days without treatment (P = 0.58). Meanwhile, postural hypotension was reported in 10 patients in the celiac group versus 8 patients in the splanchnic group (P = 0.57). Eighteen patients suffered from transient backache, which was managed by non-steroidal analgesia in the celiac group versus 16 patients in the splanchnic group (P = 0.6). Shoulder pain was the transient complaint for 4 patients in Group I versus 2 patients in Group II (P = 0.38). No major complications were reported after the procedure.
There was significant improvement on the global scale in Group II versus Group I at the 2
nd, 4
th, 6
th, and 8
th weeks (with
P values of 0.01, 0.04, 0.04, and 0.01, respectively), as shown in
Fig. 3A, while there was a significant improvement on the symptom scale in Group II versus Group I at the 16
th and 24
th weeks (with
P values of 0.02 and 0.008, respectively), as shown in
Fig. 3B. Meanwhile, physical scales improved significantly in Group II versus Group I at the 4
th and 24
th weeks (
P values = 0.04 and 0.03, respectively), as shown in
Fig. 3C. Also, there was a significant improvement on the emotional scales in Group II versus Group I at the 4
th and 24
th weeks (
P values = 0.02 and 0.01, respectively), as shown in
Fig. 3D.
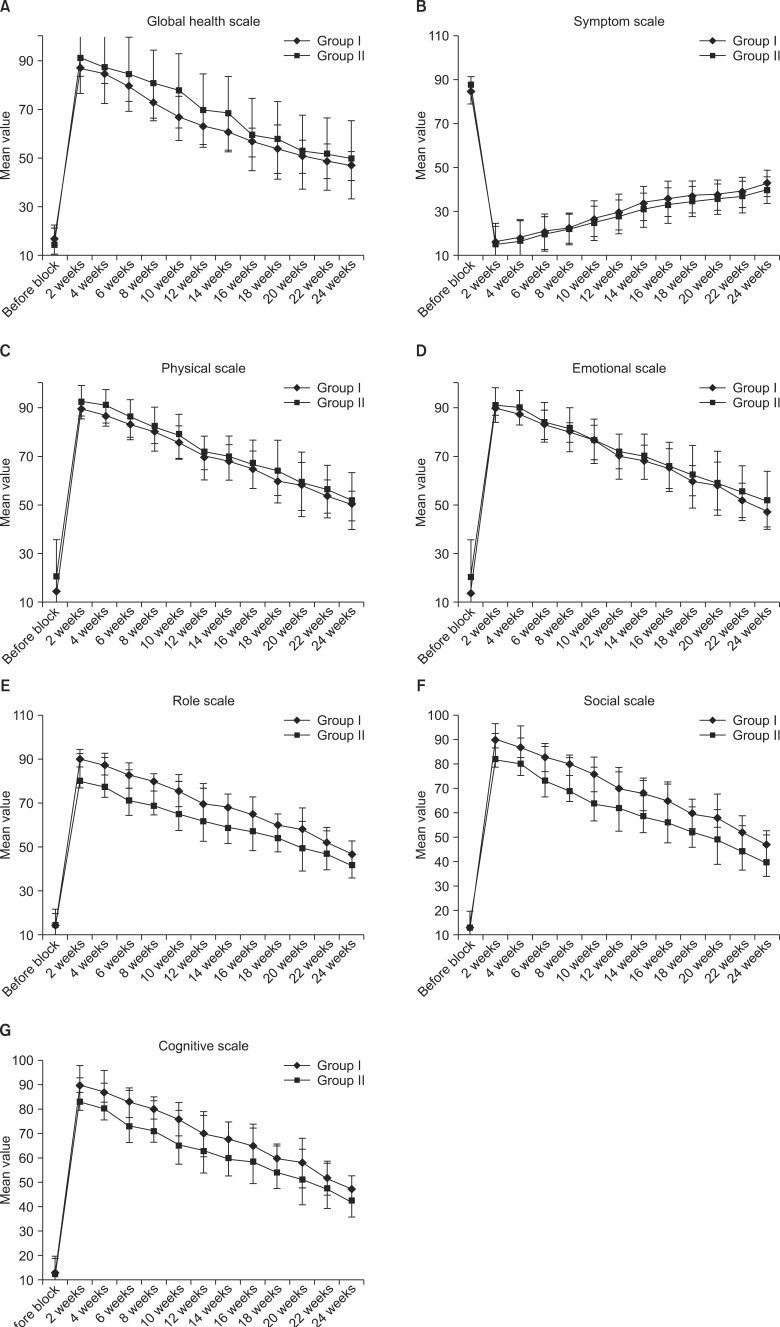 | Fig. 3(A-G) Comparison between QLQ C-30 scales before the block and during the follow-up periods for both groups. Data were presented as means ± standard deviations. 
|
There was a significant improvement on the role scales in Group II versus Group I at the 4
th and 24
th weeks (
P values = 0.03, 0.04, respectively), as shown in
Fig. 3E. However, the social scale improved significantly in Group II versus Group I from the second week onwards (
P values = 0.006, 0.03, 0.02, 0.006, 0.03, 0.02, 0.008, 0.02, 0.03, 0.003, 0.02, 0.04, in each assessment time respectively), as shown in
Fig. 3F. Also, the cognitive scales improved significantly in Group II versus Group I from the second week onwards (
P values = 0.001, 0.003, 0.001, 0.002, 0.001, 0.009, 0.002, 0.02, 0.01, 0.02, 0.04, 0.02, in each assessment time respectively), as shown in
Fig. 3G.
Patient survival rates were comparable in the two groups (
P value = 0.591), as shown in
Fig. 4.
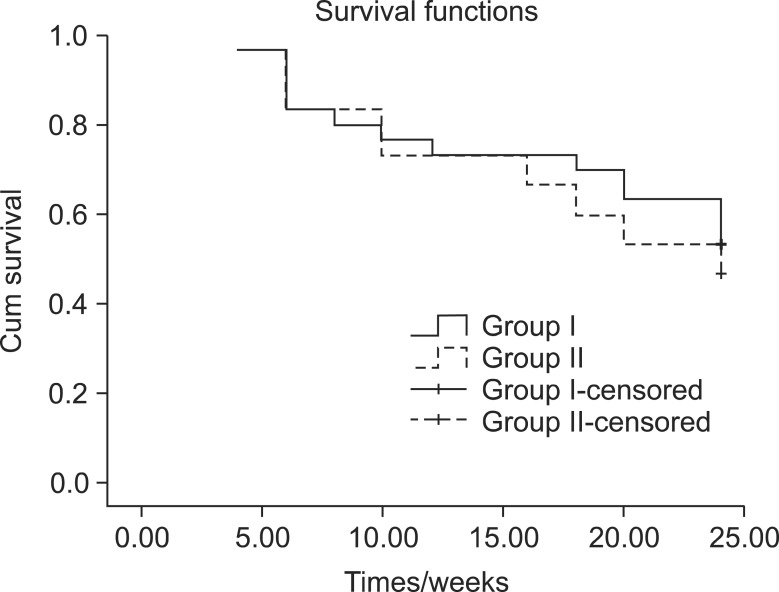 | Fig. 4Kaplan Meier curve for patient survival rates of both groups (P value = 0.591). 
|
Go to :

DISCUSSION
The majority of nociceptive impulses from the upper abdominal viscera pass through the splanchnic nerves and celiac plexus. Thus, they are perfect targets for a block for pain management, and the celiac plexus blockade is the most widely used interventional procedure for abdominal pain relief. It has also been evaluated in many studies [
10,
11,
12].
Recently, the thoracic splanchnic nerve block has gained renewed interest because the thoracic splanchnic nerve exists in a less variable anatomical relationship with surrounding structures, as it lies in a small triangular space with well-defined landmarks and boundaries and is therefore easier to reach compared to the conventional celiac plexus block [
13].
The present study shows that a splanchnic nerve block during cases of upper GIT cancer has superior results compared to a celiac plexus block, as more patients retained a good analgesic response on a weak opioid from 16 weeks onwards with improvement of the social and cognitive subscales on quality of life assessments.
Decreased opioid consumption may improve the quality of life by enhancing the immune system, as it was found that opioids have a negative effect on cellular levels [
14]. Also, a decreased sedative effect of opioids [
11,
12] was reflected in the results in the present study by the significant improvement on the social and cognitive scales in the splanchnic group versus the celiac group. Although this result was statistically significant, the importance of this has little clinical value. Both groups showed a significant reduction of opioid usage in comparison with pre-enrollment levels. An influence of opioids on the escalation of adverse gastrointestinal symptoms has been reported [
15].
In the current study, QLQ deteriorated in both groups over time; moreover, the VAS values and opioid consumption levels increased. This deterioration can be explained in terms of the progression of the tumor, a reduction in the efficacy of the neurolytic block over time, and cancer-related complications. As a consequence of the cancer-related symptoms, differences in the emotional, physical and role scales are of little clinical value.
In agreement with the current study, Stefaniak et al. [
16] compared the effectiveness of three approaches for the management of pain. Their study involved groups which received a neurolytic celiac plexus block, videothoracoscopic splanchnicectomy, and a conservative treatment as a control. Their results revealed that both of the invasive pain treatment methods resulted in significant reductions of pain and fatigue. However, they also found that a neurolytic celiac plexus block improved physical, emotional and social well-being more than a splanchnic block.
Lillemoe et al. [
17] showed that patients with unresectable pancreatic cancer receiving splanchnic neurolysis had longer survival rates. However, in our study, we did not observe a difference in the survival rates between the two neurolytic procedures.
In the present study, mortality for both procedures was nil, and complications with both techniques tended to be of minor importance apart from backache, orthostatic hypotension and self-limiting diarrhea. There have been many reports of several complications after both techniques, such as paraplegia, [
18] pneumothorax, and sexual dysfunction [
19]. Also, Davies [
20] reported that the overall incidence of major complications (e.g., paraplegia, bladder, and bowel dysfunction) was 1 in 683 procedures. Orthostatic hypotension occurred in 50% of patients who had a retrocrural celiac plexus block and in 52% of patients who had a splanchnic nerve block. In the present study, however, the incidence of hypotension was 33% in the celiac group and 29% in the splanchnic group. Transient diarrhea was frequent with the retrocrural celiac plexus block, at about 27% versus 5% in those with a splanchnic nerve block. In contrast, in the present study, transient diarrhea was reported at a rate of 34% in the celiac group and 30% in the splanchnic group. However, these reports revealed that the performance of a block with image guidance and after an injection of a local anesthetic prior to the injection of the neurolytic agent potentially reduces the risk of such complications.
There have been many reports about splanchnic nerve blocks which have been done surgically or percutaneously with neurolytic agents or with a radiofrequency method. These were found to be effective in the treatment of pain due to pancreatic cancer [
21,
22,
23]. One of the earliest studies performed to evaluate the effectiveness of a splanchnic nerve block, by Raj et al. [
22] involving 107 patients with abdominal pain of malignant and non-malignant origins, revealed good to excellent results in 55-70% of patients for pain scores, but no information was given regarding the quality of life.
Meanwhile, Süleyman Ozyalçin et al. [
24] evaluated the efficacy of celiac plexus versus splanchnic nerve neurolysis in patients with pancreatic cancer pain and revealed that splanchnic nerve neurolysis led to significantly better pain relief, quality of life, and analgesic consumption until the end of the patients' lives. However, differences in the efficacy levels of the splanchnic over the celiac group in our study as compared to the aforementioned study may be attributed to certain inclusion criteria, as the present study included all patients with upper GIT tumors (stomach, biliary tract and pancreas), while that study included patients with cancer of the pancreas, for whom pain relief is a well-known target for neurolytic celiac and splanchnic blocks.
Also, Marra et al. [
25] compared both neurolytic methods and found that the application of a splanchnic nerve block under the guidance of computed tomography produced more effective pain relief than a celiac plexus block. Meanwhile, Gangi et al. [
26] noted that a splanchnic nerve block requires a smaller volume of alcohol and has indications similar to those for a celiac plexus block.
The results of the present study demonstrate that a splanchnic nerve block appears to be clinically comparable to a celiac block, though all statistically significant differences were of little clinical value. Based on the results of the present study, we recommend further studies with longer follow-up periods, with more patients. This would increase the the potential enough to find statistically significant differences if they exist.
Go to :






 PDF
PDF Citation
Citation Print
Print









 XML Download
XML Download