Abstract
Background
Ketorolac has been used as a postoperative analgesia in combination with opioids. However, the use of ketorolac may produce serious side effects in vulnerable patients. Propacetamol is known to induce fewer side effects than ketorolac because it mainly affects the central nervous system. We compared the analgesic effects and patient satisfaction levels of each drug when combined with fentanyl patient-controlled analgesia (PCA).
Methods
The patients were divided into two groups, each with n = 46. The patients in each group were given 60 mg of ketorolac or 2 g of propacetamol (mixed with fentanyl) for 10 minutes. The patients were then given 180 mg of ketorolac or 8 g of propacetamol (mixed with fentanyl and ramosetron) through PCA. We assessed the visual analogue pain scale (VAS) at the time point immediately before administration (baseline) and at 15, 30, and 60 minutes, and 24 hours after administration. Also, the side effects of each regimen and each patient's degree of satisfaction were assessed.
Go to : 
Postoperative pain is an aggravating problem. It can be persistent and involve impaired rehabilitation, an increased length of hospital stay and/or readmission, and can have adverse events related to excessive analgesic use, such as nausea. Opioids are the first line of therapy for patients with moderate to severe postoperative pain. However, these drugs do not always provide adequate analgesia and their use is associated with dose-related side effects [12]. For these reasons, non-opioid agents are usually added to enhance the analgesic effects while reducing the opioid-induced side effects.
Non-steroidal anti-inflammatory drugs (NSAIDs) are typical non-opioid analgesics. The analgesic effect of NSAIDs can be explained in terms of the peripheral inhibition of cyclooxygenase 1 or 2 (COX-1, COX-2), which reduces postoperative opioid consumption and improves the analgesic quality [345]. Ketorolac tromethamine (ketorolac), a heterocyclic derivative of acetic acid, has been used for postoperative analgesia in combination with opioids. Several studies have reported that ketorolac is as effective as morphine or meperidine for analgesia after some types of surgical procedures [67]. However, because many studies report significant side effects of ketorolac, including coagulopathy, gastrointestinal problems, and nephrotoxicity [89], there is increasing interest in the use of other classes of non-opioid analgesics.
Propacetamol, an injectable prodrug of acetaminophen, is the most commonly prescribed analgesic for the treatment of acute pain in North America [10]. Its advantage over NSAIDs is its lack of interference with platelet functions. Moreover, it is safe to administer to patients with a history of peptic ulcers or asthma [11]. Its mechanism of action may involve a central inhibition of COX-2 [1213], inhibition of nitric oxide generation via a blockade of the N-methyl-D-aspartate (NMDA) receptor [14], and activation of the descending serotonergic pathway [15]. Propacetamol can cross the blood-brain barrier, producing a central analgesic effect [16]. Thanks to this mechanism, it is known to have fewer side effects than ketorolac.
We compared analgesic effects, side effects, and each patient's level of satisfaction for both non-opioids combined with fentanyl through an intravenous patient-controlled analgesic application in postoperative patients.
Go to : 
The protocol was approved by the hospital's Institutional Review Board (Chonnam National University Medical School, Jebongro 42, Dong-gu, Gwangju 501-191, Korea, 16 May 2013, protocol number CNUH-2013-083) and written informed consent was obtained from each patient pre-operatively. We used a randomized, active-controlled, open-label design in our single-center trial. The study took place at the anesthesiology and pain medicine department of Chonnam National University Hospital in Gwang-ju, Korea from May to September 2013.
Ninety-two patients, aged 20-70 years undergoing elective abdominal (including laparoscopic) and gynecologic surgery with general anesthesia were enrolled in the study. Patients were excluded if they were affected by severe hepatic, renal, or gastric disease; if they were given additional analgesics, anti-inflammatory drugs, or antipyretic drugs during the study; or if they had contraindications to ketorolac, paracetamol, or fentanyl. Patients were randomized into two groups of 46 patients each using a computer-generated table. All patients received 0.125-0.25 mg of triazolam as a premedication. General anesthesia was induced using a combination of propofol (2 mg/kg), rocuronium (0.8 mg/kg), and a remifentanil infusion. Anesthesia was maintained with a combination of sevoflurane and O2 (FiO2: 0.5, 3 L/min), and the BIS score was maintained within the range of 40-60. The end-tidal carbon dioxide (ETCO2) level was maintained at 40 mmHg. A reversal agent was injected just before the end of the operation, and 0.3 mg of ramosetron (Nasea®, Ramosetron HCL, Astellas Korea) was given to prevent postoperative nausea and vomiting. After recovering spontaneous respiration, the patients were moved to the recovery room.
The baseline pain score was assessed just after the patients became alert and could recognize their names clearly. Ketorolac (Keromin®, Ketorolac tromethamine 30 mg/ml, Hana medical, Korea) or propacetamol (Denogan®, Propacetamol HCL 1 g/ample, Young-Jin medical, Korea) was administered using a random-order sheet when the patients requested analgesia. The patients in the ketorolac group were administered 60 mg of ketorolac (mixed with 100 µg of fentanyl to a total volume of 100 ml) for 10 minutes. Additionally, the patients were administrated 180 mg of ketorolac, 1000 µg of fentanyl, and 0.6 mg of ramosetron (mixed in saline, to a total volume of 100 ml) through a patient-controlled analgesic application (PCA, continuous for 1.5 ml/hr, bolus of 1.5 ml, lock out time of 10 minutes). The patients in the propacetamol group were given 2 g of propacetamol (mixed with 100 µg of fentanyl to a total volume of 100 ml) for 10 minutes. Additionally, 8 g of propacetamol, 1000 µg of fentanyl, and 0.6 mg of ramosetron (mixed in saline, to a total volume of 100 ml) were administered via PCA in the same manner. When patients requested additional analgesics in the recovery room, an amount of 50 mg of tramadol (VAS 5-6) or 12.5-25 mg of meperidine (VAS above 6) was administered. Additionally, 10 mg of macperan (metoclopramide, Jeil Pharmaceutical Co., Ltd., Korea) was given within the first 6 hours, and 0.3 mg of ramosetron was given after the first 6 hours to manage postoperative nausea and vomiting. Pain intensity levels were subjectively measured using a 10 cm visual analogue pain scale (VAS, 0 = no pain to 10 = unbearable pain). We assessed VAS, the infused volumes of drugs, vital signs (mean blood pressure and heart rate) and the side effects of the each regimen immediately before administration (baseline) and at 15, 30, and 60 minutes, and at 24 hours after administration. During drug administration, the presence of injection pain was also assessed. Further, each patient's degree of satisfaction was assessed using the pain treatment satisfaction scale (PTSS, Fig. 1) at 24 hours after administration [17].
Zhou et al. [18] compared changes in postoperative VAS scores of patients with ketorolac and saline. The VAS score change in the ketorolac group was 3.03 (± 2.77) while in the saline group it was 1.41 (± 2.18). The difference in the change in the VAS score between the ketorolac group and the saline group was 1.62. The determined non-inferiority margin in the present study was 1.2. The sample size calculation suggested 42 subjects per group (a parallel design with α = 0.05, power = 0.8, σ = 2.18, and δ = 1.2 for pain relief scores). Considering the failure rate, 50 subjects in each group participated in the present study (added 18% to the sample size). SPSS version 15.0 for Windows was used for statistical evaluations. The Mann-hitney U-test was used to compare demographic data, VAS scores, vital signs, and side effects. Repeated measurements were tested within groups using Friedman's ANOVA and the Wilcoxon rank sum test. Values were expressed as means ± standard deviations. P < 0.05 was accepted as significant except for the repeated measurements, for which the accepted significance threshold was P < 0.01.
Go to : 
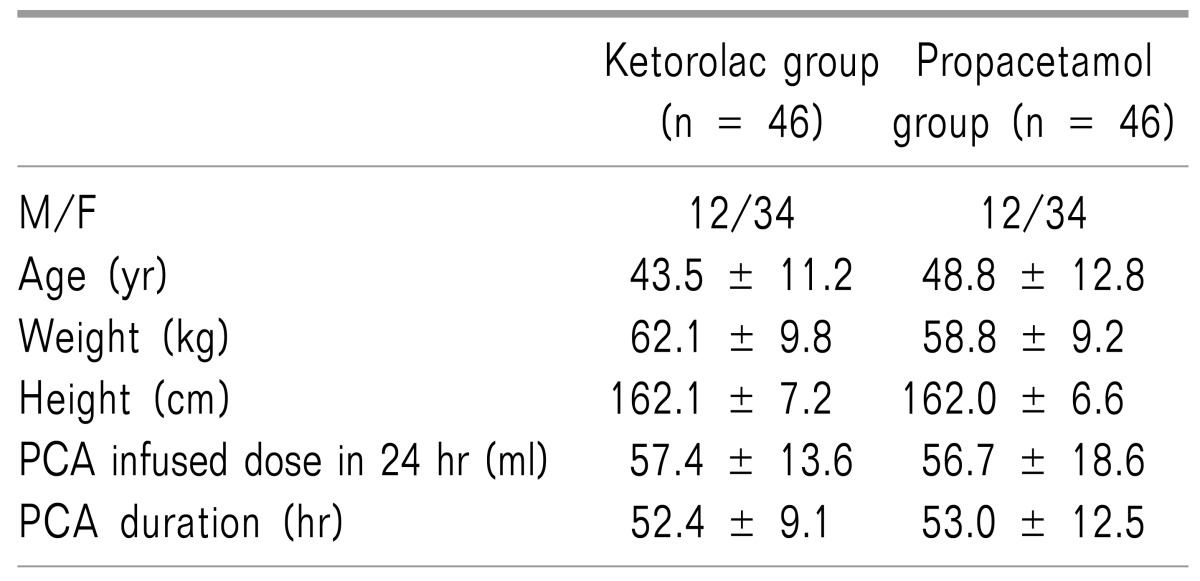
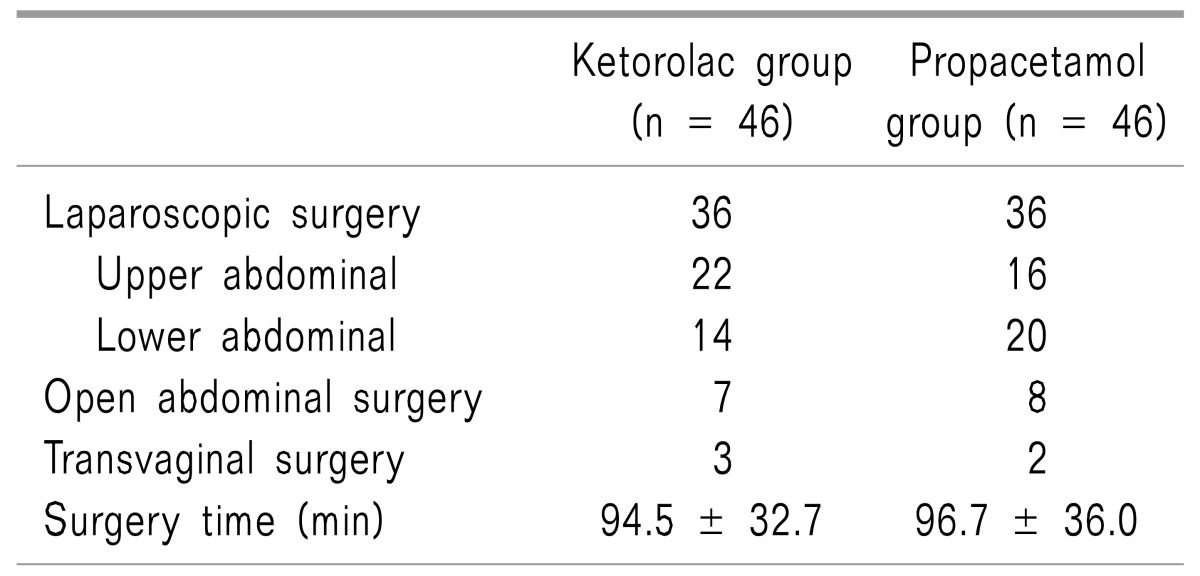
The demographic data of the two groups was similar, and there were no significant differences in the surgery time, initial pain intensity, PCA administration dose over 24 hours or the total maintain time (Table 1). Although 100 patients were initially randomized into the study groups, eight patients were excluded from the main analysis, six due to a protocol violation, and two owing to an equipment failure (Fig. 2). The types of surgery in each group are presented in Table 2.
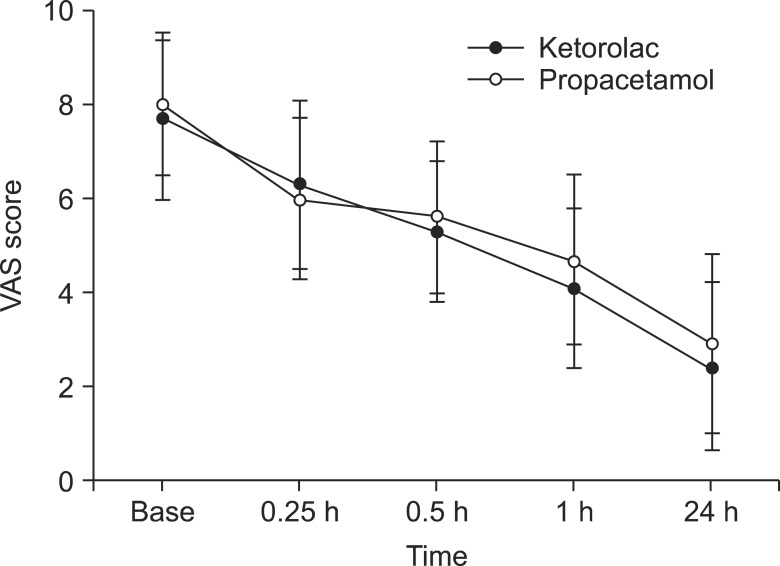
Compared with baseline scores, there were significant declines in VAS scores in both groups throughout the time sequence (P < 0.05). The statistical VAS score was slightly higher in the ketorolac group at most time points, except for the time of 15 min. However, the differences between groups were not statistically significant (Fig. 3) and the values were within the determined non-inferiority margin at all of the time points. Also, there were no significant differences in vital signs (MBP, HR) between the groups.
There were no significant differences in VAS scores and vital signs, or satisfaction scores for different types of surgery between the groups.
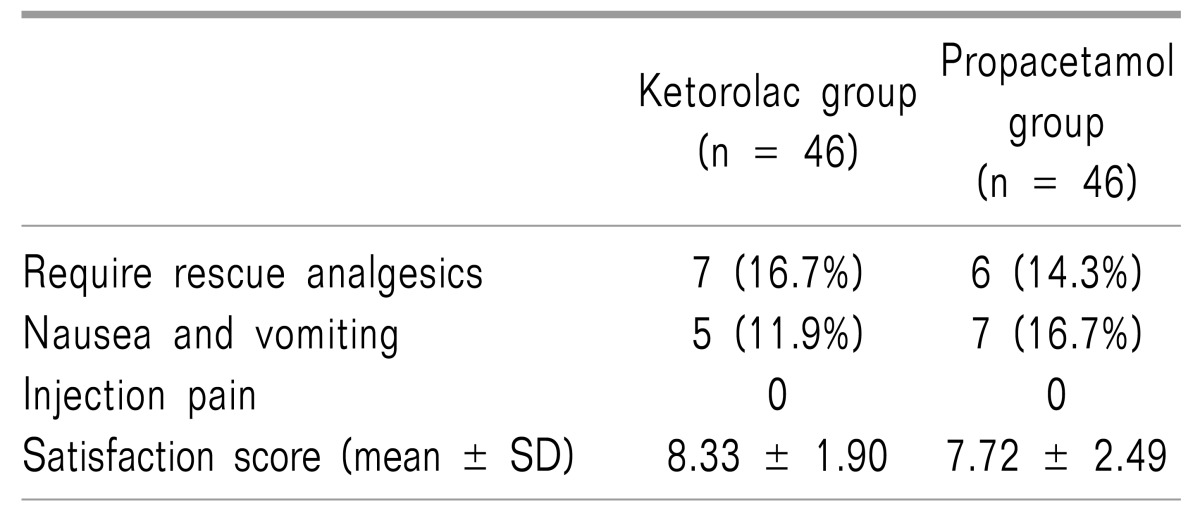
The side effects and satisfaction scores for each group are presented in Table 3. Seven patients wanted an additional analgesic (16.7%) in the ketorolac group. Six (14.3%) requested an analgesic in the propacetamol group. All patients who requested additional analgesia in the ketorolac group and four patients in the propacetamol group received 50 mg of tramadol intravenously. Two patients in the propacetamol group received 12.5 mg of meperidine. Five patients complained of nausea (11.9%) in the ketorolac group and seven did so (16.7%) in the propacetamol group. Interestingly, female patients made the majority of nausea complaints in both groups. Only one male patient in the ketorolac group complained of nausea. Injection pain was not reported in either group.
Satisfaction scores were statistically higher in the ketorolac group (8.33 ± 1.90 in the ketorolac group, 7.72 ± 2.49 in the propacetamol group), but an analysis between the groups showed that the difference was not significant. No serious adverse drug reactions were reported in either group.
Go to : 
Propacetamol, a prodrug of paracetamol, is hydrolyzed to acetaminophen and diethylglycerine at a 1:1 ratio by plasma esterase within seven minutes after administration. Therefore, a dose of 2 g of propacetamol yields 1 g of acetaminophen after hydrolysis. There are many reports that compare the efficacy of acetaminophen and ketorolac. McQuay et al. [19] reported that 1 gram of oral acetaminophen was similar to 10-20 mg of ketorolac taken orally for pain relief after orthopedic surgery. Another report suggests that a combination of 2 g of propacetamol and 30 mg of ketorolac has a similar morphine-sparing effect with intravenous PCA after gynecologic surgery [20].
In this study, we compared the analgesic efficacy of 8 g of propacetamol and 180 mg of ketorolac as a PCA dose. Despite the relatively low dose of propacetamol, as compared to that of ketorolac, the analgesic efficacy of the propacetamol group was comparable to that of the ketorolac group in postoperative patients using patient-controlled analgesia with fentanyl. This suggests that propacetamol is effective when used for the management of postoperative pain and combined with fentanyl PCA.
Several previous studies have examined the analgesic efficacy of ketorolac and propacetamol in combination with patient-controlled analgesia morphine after gynecologic surgery [20]. Compared to morphine, fentanyl is approximately 100 times more potent, as 0.1 mg of fentanyl is approximately equivalent to 10 mg of morphine and 75 mg of pethidine (meperidine) in terms of its analgesic activity. Despite being a more potent analgesic, fentanyl tends to induce less nausea and constipation, as well as less histamine-mediated itching, in comparison with morphine [21]. For these reasons, we use fentanyl instead of morphine as the opioid in this study.
PCA with intravenous opioids is a well-established technique for postoperative pain control after major surgery. This technique is advantageous, as it is easier to adjust the level of analgesia compared to intravenous bolus doses and because is increases patient satisfaction and cooperation [22]. However, postoperative pain management is often limited by the side effects of opioids; especially when used alone in large doses for an extensive period, opioids can lead to acute tolerance [23] and, more seriously, respiratory depression and hypotension [24]. For these reasons, multimodal approaches that add non-opioid agents to opioid-based regimens are favorable. In many reports, the use of ketorolac as an adjuvant to a PCA opioid resulted in an opioid-sparing effect ranging from 16% to 33% [25262728].
However, there are risks when adding ketorolac as an adjuvant to an opioid for postoperative pain management. Gastrointestinal bleeding, platelet dysfunction, and renal impairment are adverse effects associated with the administration of ketorolac. The incidence of serious adverse events has declined since the dosage guidelines were revised [29]. Most of the published literature suggests that the overall risk of gastrointestinal or operative-site bleeding related to the administration of ketorolac is inappreciable or only slightly higher than that of opioids [303132]. Nevertheless, the risk of adverse effects increases with prolonged therapy, high doses of ketorolac, and in vulnerable patients (e.g., the elderly) [333435].
In comparison with ketorolac, propacetamol has minimal adverse effects when used for postoperative pain management [36]. In addition, propacetamol is known to have a shorter analgesic time than ketorolac. Zhou et al. [18] reported that 2 g of propacetamol has a shorter analgesic onset time than 15 mg or 30 mg of ketorolac in patients after total knee or hip replacement surgery (8 minutes with 2 g of propacetamol and 14 and 10 minute in 15 mg and 30 mg of ketorolac, respectively). The analgesic efficacy of propacetamol was comparable to ketorolac in this study. Therefore, it is reasonable to use propacetamol, instead of ketorolac, with fentanyl PCA in high-risk patients requiring postoperative analgesia.
However, there are some risks associated with the use of propacetamol. Acute overdoses of paracetamol can cause potentially fatal hepatic failures. The level of risk may be increased in chronic alcohol drinkers. Also, propacetamol requires reconstitution; in some cases, allergic contact dermatitis has been observed in those who handled the drug. Additionally, it may cause pain at the site of injection, although this discomfort can be reduced by a slow infusion [37]. In this study, hepatic failure and injection pain were not reported, and no instance of allergic dermatitis arose.
There are several limitations in the present study. First, we used a relatively high dose of fentanyl (total 1100 µg, bolus 100 µg, PCA 1000 µg). It is possible that the comparable VAS scores in the two groups stemmed from the potent analgesic effect of fentanyl. We cannot eliminate this possible confounding variables because we did not include a control group which solely used fentanyl. Second, the causes of nausea are uncertain. Many factors can induce postoperative nausea and vomiting. For example, severe postoperative pain, opioids, and rescue drugs (tramadol or meperidine) can cause nausea. Two patients complained of nausea and severe pain concurrently in the propacetamol group, and three in the ketorolac group. These patients all received 25 mg of meperidine as a rescue drug. The cause of postoperative nausea could vary under these conditions. Third, there are several faults in the study protocol. In the sample size calculation, the non-inferiority margin determined in this study was higher than the generally accepted level. Moreover the inclusion criteria for the surgery type were too broad to verify the outcomes. The majority of patients in both group underwent laparoscopic surgery. Consequently, there are unclear aspects with regard to the comparable analgesic efficacy of the drugs in cases of open abdominal or transvaginal surgery due to the small sampling size. Also, the number of those who underwent upper abdominal laparoscopic surgery was higher in the ketorolac group than in the propacetamol group. This factor can affect the outcome of the study because it is commonly known that upper abdominal surgery can induce more severe postoperative pain than lower abdominal surgery. Finally, there was a lack of safeguards in the setting of the PCA program. Theoretically, it is possible to infuse up to 252 ml of a drug in 24 hours in the PCA setting used in this study. Fortunately, no patients received such an overdose in this study. Nevertheless, we should have set additional parameters for safety, such as a four-hour dose limitation.
In spite of these limitations, we feel that propacetamol is an effective non-opioid analgesic agent that offers a useful alternative to NSAIDs for the management of postoperative pain owing to its well-known safety profile.
Go to : 
References
1. Weis OF, Sriwatanakul K, Alloza JL, Weintraub M, Lasagna L. Attitudes of patients, housestaff, and nurses toward postoperative analgesic care. Anesth Analg. 1983; 62:70–74. PMID: 6129821.

2. Melzack R, Abbott FV, Zackon W, Mulder DS, Davis MW. Pain on a surgical ward: a survey of the duration and intensity of pain and the effectiveness of medication. Pain. 1987; 29:67–72. PMID: 3588001.

3. Moote C. Efficacy of nonsteroidal anti-inflammatory drugs in the management of postoperative pain. Drugs. 1992; 44(Suppl 5):14–29. PMID: 1284558.

4. Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991; 66:703–712. PMID: 2064886.

5. Fredman B, Olsfanger D, Jedeikin R. A comparative study of ketorolac and diclofenac on post-laparoscopic cholecystectomy pain. Eur J Anaesthesiol. 1995; 12:501–504. PMID: 8542859.
6. O'Hara DA, Fragen RJ, Kinzer M, Pemberton D. Ketorolac tromethamine as compared with morphine sulfate for treatment of postoperative pain. Clin Pharmacol Ther. 1987; 41:556–561. PMID: 3568540.
7. Yee JP, Koshiver JE, Allbon C, Brown CR. Comparison of intramuscular ketorolac tromethamine and morphine sulfate for analgesia of pain after major surgery. Pharmacotherapy. 1986; 6:253–261. PMID: 3540877.

8. Cashman J, McAnulty G. Nonsteroidal anti-inflammatory drugs in perisurgical pain management. Mechanisms of action and rationale for optimum use. Drugs. 1995; 49:51–70. PMID: 7705216.

9. Kenny GN. Potential renal, haematological and allergic adverse effects associated with nonsteroidal anti-inflammatory drugs. Drugs. 1992; 44(Suppl 5):31–36. PMID: 1284559.

10. Sachs CJ. Oral analgesics for acute nonspecific pain. Am Fam Physician. 2005; 71:913–918. PMID: 15768621.
11. Hyllested M, Jones S, Pedersen JL, Kehlet H. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. Br J Anaesth. 2002; 88:199–214. PMID: 11878654.

12. Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005; 12:46–55. PMID: 15662292.

13. Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005; 94:505–513. PMID: 15681586.

14. Björkman R, Hallman KM, Hedner J, Hedner T, Henning M. Acetaminophen blocks spinal hyperalgesia induced by NMDA and substance P. Pain. 1994; 57:259–264. PMID: 7524008.

15. Bonnefont J, Courade JP, Alloui A, Eschalier A. Antinociceptive mechanism of action of paracetamol. Drugs. 2003; 63 Spec No 2:1–4. PMID: 14758785.
16. Bannwarth B, Netter P, Lapicque F, Gillet P, Péré P, Boccard E, et al. Plasma and cerebrospinal fluid concentrations of paracetamol after a single intravenous dose of propacetamol. Br J Clin Pharmacol. 1992; 34:79–81. PMID: 1633071.

17. Evans CJ, Trudeau E, Mertzanis P, Marquis P, Peña BM, Wong J, et al. Development and validation of the Pain Treatment Satisfaction Scale (PTSS): a patient satisfaction questionnaire for use in patients with chronic or acute pain. Pain. 2004; 112:254–266. PMID: 15561380.

18. Zhou TJ, Tang J, White PF. Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesth Analg. 2001; 92:1569–1575. PMID: 11375848.

19. McQuay HJ, Poppleton P, Carroll D, Summerfield RJ, Bullingham RE, Moore RA. Ketorolac and acetaminophen for orthopedic postoperative pain. Clin Pharmacol Ther. 1986; 39:89–93. PMID: 3510797.

20. Varrassi G, Marinangeli F, Agrò F, Aloe L, De Cillis P, De Nicola A, et al. A double-blinded evaluation of propacetamol versus ketorolac in combination with patient-controlled analgesia morphine: analgesic efficacy and tolerability after gynecologic surgery. Anesth Analg. 1999; 88:611–616. PMID: 10072016.

21. Mayes S, Ferrone M. Fentanyl HCl patient-controlled iontophoretic transdermal system for the management of acute postoperative pain. Ann Pharmacother. 2006; 40:2178–2186. PMID: 17164395.

22. Kollender Y, Bickels J, Stocki D, Maruoani N, Chazan S, Nirkin A, et al. Subanaesthetic ketamine spares postoperative morphine and controls pain better than standard morphine does alone in orthopaedic-oncological patients. Eur J Cancer. 2008; 44:954–962. PMID: 18396035.

23. Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995; 62:259–274. PMID: 8657426.

24. Tveita T, Thoner J, Klepstad P, Dale O, Jystad A, Borchgrevink PC. A controlled comparison between single doses of intravenous and intramuscular morphine with respect to analgesic effects and patient safety. Acta Anaesthesiol Scand. 2008; 52:920–925. PMID: 18702754.

25. Parker RK, Holtmann B, Smith I, White PF. Use of ketorolac after lower abdominal surgery. Effect on analgesic requirement and surgical outcome. Anesthesiology. 1994; 80:6–12. PMID: 8291731.

26. Sevarino FB, Sinatra RS, Paige D, Ning T, Brull SJ, Silverman DG. The efficacy of intramuscular ketorolac in combination with intravenous PCA morphine for postoperative pain relief. J Clin Anesth. 1992; 4:285–288. PMID: 1419009.

27. Rogers JE, Fleming BG, Macintosh KC, Johnston B, Morgan-Hughes JO. Effect of timing of ketorolac administration on patient-controlled opioid use. Br J Anaesth. 1995; 75:15–18. PMID: 7669460.

28. Ready LB, Brown CR, Stahlgren LH, Egan KJ, Ross B, Wild L, et al. Evaluation of intravenous ketorolac administered by bolus or infusion for treatment of postoperative pain. A double-blind, placebo-controlled, multicenter study. Anesthesiology. 1994; 80:1277–1286. PMID: 8010474.

29. Gillis JC, Brogden RN. Ketorolac. A reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997; 53:139–188. PMID: 9010653.
30. Koh SC, Pua HL, Tay DH, Ratnam SS. The effects of gynaecological surgery on coagulation activation, fibrinolysis and fibrinolytic inhibitor in patients with and without ketorolac infusion. Thromb Res. 1995; 79:501–514. PMID: 7502276.

31. Varrassi G, Panella L, Piroli A, Marinangeli F, Varrassi S, Wolman I, et al. The effects of perioperative ketorolac infusion on postoperative pain and endocrine-metabolic response. Anesth Analg. 1994; 78:514–519. PMID: 8109770.

32. Strom BL, Berlin JA, Kinman JL, Spitz PW, Hennessy S, Feldman H, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding. A postmarketing surveillance study. JAMA. 1996; 275:376–382. PMID: 8569017.

33. Henry D, Lim LL, Garcia Rodriguez LA, Perez Gutthann S, Carson JL, Griffin M, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 1996; 312:1563–1566. PMID: 8664664.

34. García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual nonsteroidal anti-inflammatory drugs. Lancet. 1994; 343:769–772. PMID: 7907735.

35. Cummings DM, Amadio P Jr. A review of selected newer nonsteroidal anti-inflammatory drugs. Am Fam Physician. 1994; 49:1197–1202. PMID: 8154406.
36. White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002; 94:577–585. PMID: 11867379.

37. Depré M, van Hecken A, Verbesselt R, Tjandra-Maga TB, Gerin M, de Schepper PJ. Tolerance and pharmacokinetics of propacetamol, a paracetamol formulation for intravenous use. Fundam Clin Pharmacol. 1992; 6:259–262. PMID: 1487229.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download