Abstract
Background
Lack of proper control of acute postoperative pain often leads to lingering or chronic pain. Several studies have emphasized the role of beta-blockers in reducing postoperative pain. Esmolol is a selective short-acting beta-blocker that produces few side effects. The purpose of this study was to examine the effect of intravenous intraoperative esmolol on postoperative pain reduction following orthopedic leg fracture surgery.
Methods
In a clinical trial, 82 patients between 20-65 years of age with tibia fractures and American Society of Anesthesiologists (ASA) physical status I & II who underwent surgery were divided into two groups. Group A received esmolol and group B received normal saline. Postoperative pain was measured at three time points: entering the recovery unit, and at 3 h and 6 h following surgery, using the Visual Analogue Scale (VAS). A P value of < 0.05 was considered significant.
Results
Mean VAS scores at all three time points were significantly different between the two test groups (P = 0.02, P = 0.0001, and P = 0.0001, respectively). The consumption of pethidine was lower in group A than in group B (P = 0.004) and the duration of its effect was significantly longer in time (P = 0.026).
Go to : 
Beta-blockers have recently been investigated for the management of postoperative pain. These drugs are increasingly being used to reduce perioperative cardiac complications [1]. There is evidence that beta-blockers reduce neuronal stimulation responses of the cingulate cortex in rats. The analgesic effects of these drugs have been reported in rats and in the treatment of allodynia in humans [2]. Beta-blockers are also used to reduce the stress response and decrease the need for narcotic drugs following surgery [3].
A new trend in the reduction of pain using beta-blockers has developed. It has been shown that esmolol, as a selective short-acting antagonist, has analgesic effects in addition to cardiovascular effects. For example, the study by Ono and colleagues showed that intrathecal administration of esmolol had analgesic effects on postoperative pain in animals [4]. Bhawna and his colleagues showed that esmolol can reduce the need for opioids after abdominal surgery [5].
It appears that beta-blockers may have beneficial effects during surgery including stabilizing the patient's intraoperative hemodynamic status and may also result in reduced postoperative opioid consumption [16]. However, few studies have focused solely on the analgesic effects of esmolol, and no previous study has examined the analgesic effects of esmolol in orthopedic surgery. Patients undergoing orthopedic surgery represent a high percentage of cases treated in Iranian hospitals. Methods that reduce postoperative pain will decrease complications, increase patient comfort, and reduce overall hospital costs. With these goals in mind, our study was designed to determine the effect of intravenous intraoperative esmolol on pain reduction following lower limb orthopedic surgery.
Go to : 
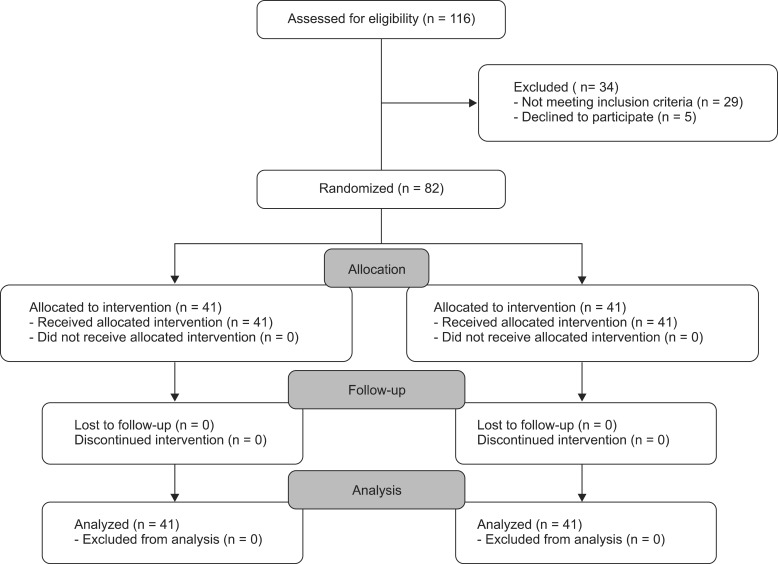
This randomized controlled study was performed on 82 patients treated for leg fractures (American Society of Anesthesiologists [ASA] physical status class I & II) who were admitted to Poursina Hospital in Rasht for orthopedic surgery. The study complied with ethical requirements. All patients were informed about the type of medication prescribed and possible side effects and written informed consent was obtained from each participant (Fig. 1).
Inclusion criteria consisted of all patients between 20 and 60 years of age who had a single leg displaced fracture of the tibia and who were scheduled to undergo surgical fixation using a plate and screws under general anesthesia (with isoflurane). Exclusion criteria eliminated patients with a body mass index (BMI) outside the range of 20-40; patients with heart, liver, lung or kidney disease; patients with a history of allergy to opioid medications; patients who regularly or within three days before the scheduled surgery had received any analgesic (e.g., NSAIDs, opioids, or paracetamol); and patients with a known history of psychiatric disease, regular use of psychiatric drugs, or addiction to opioids.
Patients were admitted consecutively into the recovery room and divided into two groups based on random blocks of four. Group A received esmolol and group B received placebo. For the double-blind conditions of the study, the required infusion solution was prepared by the anesthetist with no knowledge of the Visual Analogue Scale (VAS) score and no previous contact with any of the patients. Infusions were performed by anesthesia residents who were unaware of the study groups and the infusion solutions. A loading dose of esmolol (0.5 mg/kg in 30 ml normal saline) was infused to group A 30 min before induction of anesthesia and was continued as a maintenance dose by intravenous infusion (5 µg/kg/min) until the closure of the incision. Patients in the control group (B) first received 30 cc of normal saline (the same color and size as the loading dose of esmolol administered to group A) infused over a period of 5 min, and then a saline infusion (0.005 ml/kg/min, equivalent to that of group A) was continued until the closure of the incision. Hypotension would be treated with intermittent doses of ephedrine (5 mg) and bradycardia would be treated with intermittent doses of atropine (0.5 mg). In such cases, these patients would be excluded from the study.
A questionnaire including demographic information, including age, sex and weight of the patient, and date, type and duration of the operation was prepared and completed. The VAS was determined and recorded by the anesthesia resident, first in the recovery unit and then 3 and 6 h after surgery in the orthopedic ward.
Anesthesia was administered to all patients in the operating room after the preparation and monitoring connections were completed. Induction of anesthesia was similar in all patients: 5 ml/kg of normal saline before induction, then fentanyl (2 µg/kg), midazolam (2 mg), sodium thiopental (3-5 mg/kg), and atracurium (0.5 mg/kg). During surgery, anesthesia was maintained by administration of isoflurane, nitrous oxide, and oxygen. Vital signs were recorded every 5 min during surgery. At intervals of 30 min during the operation a muscle relaxant was administered. Bispectral index (BIS) monitoring was performed to prevent patient awareness during the surgery. If BIS was increasing, the concentration of isoflurane was increased until BIS was below 60, and if BIS decreased to below 40, the isoflurane concentration was reduced. The scheduled duration of the operation for all patients was 60 to 120 min, while patients whose surgery extended outside of this time period and those whose initial surgical method was changed were excluded. In addition, patients with serious complications such as cardiac or respiratory arrest and patients who had required interventions that could affect our procedures were excluded.
The same drug (intravenous pethidine 0.5-1.5 mg/kg) was used for pain relief in all cases and was administered to patients with VAS scores above 3 after patients' self-reported discomfort. The pethidine dose prescribed was recorded on the initial questionnaire and patients were monitored for a 24 h period after surgery for hemodynamic complications (such as changes in blood pressure, heart rate, etc.) and other adverse events. Remedial measures were to be taken in the event of complications.
The sample size for comparison of pain between the two groups was estimated based on the results of a pilot study with 15 patients in each group, with a 95% confidence interval and a power of 90%. The required sample size was estimated to be 41 patients in each group. The theta coefficient for the effect size was estimated to be 0.13.
All collected data were analyzed by SPSS software (IBM SPSS Statistics, IBM Corp., Chicago, IL, USA) and tables and graphs were used to organize and summarize the information. Descriptive objectives were achieved using 95% confidence intervals. Analytical aims were achieved by initially testing the normal distribution of VAS scores by the Kolmogorov-Smirnov (KS) test, and an independent t-test was used to compare pain in the two groups according to normal distribution. To investigate the use of pethidine between the two groups, the t-test was utilized. Qualitative variables were analyzed by the chi-square test. The significance level for all tests was considered as P < 0.05.
Go to : 
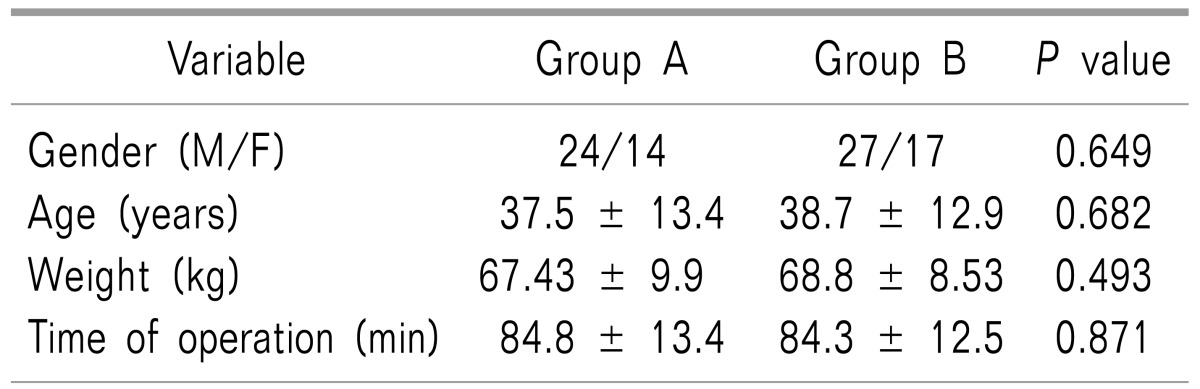
A total of 82 patients (51 male and 31 female patients) participated in this study (Fig. 1). Demographic data are shown in Table 1. No significant differences were found in sex distribution, age, weight, or duration of surgery (Table 1). There were no major complications in any of the patients, nor were any patients excluded from the study.
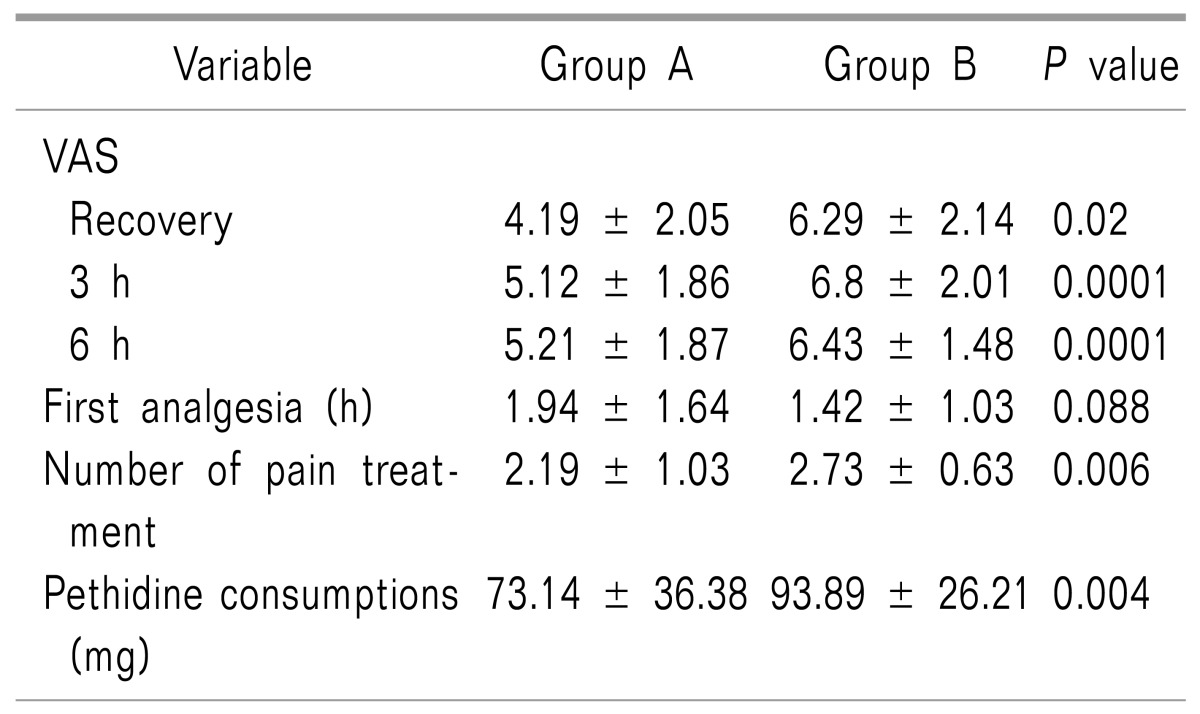
After statistical analysis using the t-test, a significant difference in pain status based on VAS criteria was observed between the two groups during the recovery period as well as at the third and sixth hours following surgery (P = 0.02, P = 0.001, and P = 0.0001, respectively, Table 2). The VAS score was lower in the esmolol group at each of these monitoring timepoints. The amount of analgesic (pethidine) that was administered to the patients after surgery was also significantly different between the esmolol group (73.14 ± 36.38 mg) and the placebo group (93.89 ± 25.21 mg) (P = 0.004). In addition, the average retention time of not receiving analgesic (up to 6 h after surgery) between the two groups showed a significant difference (P = 0.026).
Go to : 
In this study, it was shown that intravenous intraoperative infusion of esmolol has a significant effect in reducing postoperative pain and narcotic intake following surgery. Beta-blockers suppress the circulating catecholamine increase that is induced by surgical stress and thereby reduce adverse perioperative cardiovascular events. In fact, these medications reduce the input of the central nervous system to decrease perioperative pain and adjust the pathophysiology that occurs during surgery. There is also evidence that beta-blockers reduce the rat cortical neuronal excitatory responses in the cingulate cortex [2]. We found that the mean VAS score was significantly lower in the esmolol group. Our finding is similar to the results of previous studies such as that of Lee, who investigated the effect of perioperative esmolol infusion during laparoscopic appendectomy [7]. However, that study and other previous studies did not investigate VAS scores or postoperative pain, and often examined the amount of postoperative analgesic consumption (including patient controlled analgesia [PCA] or bolus narcotics). In a study by Casalino on bariatric surgery patients, intraoperative esmolol infusion reduced pain during the first 6 hours after surgery and it was significantly effective as epidural analgesia [8]. Coloma even found that intraoperative esmolol infusion was as effective as intraoperative remifentanil infusion in reducing postoperative pain in gynecologic laparoscopic surgeries [9].
In our study, the intake of the analgesic drug pethidine for the treatment of postoperative pain was significantly lower in the esmolol group than in the placebo group. Given that there was less postoperative pain (lower VAS scores) reported by the patients receiving esmolol, these two findings confirmed each other and were similar to those of previous studies on esmolol including White's study with gynecologic surgery [10], Ozturk's study on laparoscopic cholecystectomy [11], Celebi's septorhinoplasty study [12], and laparoscopic cholecystectomy studies by Collard et al. [13]. In all these studies, there was a significant decrease in postoperative opioids (morphine, fentanyl) administered to the patients [10111213]. In some studies, intraoperative beta-blockade and postoperative narcotic administration were evaluated. Stanley and his colleagues showed that the use of propranolol reduced the need for postoperative narcotics [14]. Jakobsen and his colleagues administered metoprolol orally between 10-12 hours before their patients underwent ear, nose, and septum surgery and observed that the halothane concentration required to reduce the recovery time was lower [15].
Methods that reduce postoperative pain, decreasing the administration of opioid or non-opioid analgesics, lessen the side effects of nausea, vomiting, and respiratory depression experienced by the patient. This may also reduce the costs imposed on the patient [13].
In a study conducted in 2004 by Chia, it was shown that perioperative administration of esmolol was able to reduce the use of halothane and isoflurane during surgery, and it also reduced morphine consumption during the first three days after surgery. In a 1997 study by Johansen, it was revealed that esmolol could significantly reduce the anesthetic dose required for skin incision; this mechanism was named the anesthetic-sparing effect [1516].
While the incidence of beta-blocker side effects (e.g., bradycardia, heart failure, or hypotension) is dangerous, esmolol as a short-acting drug can be more effective than long-acting oral medications. One limitation of the current study, however, is that there were no esmolol plasma level measurements. We also suggest that further studies should be done to investigate postoperative pain for a longer period of time (24-72 h) and to compare esmolol with other beta-blockers such as metoprolol.
In conclusion, our study showed that there was a reduction in opioid intake in the esmolol group compared to the placebo group. Furthermore, the duration of analgesia in the postoperative period was also longer in the esmolol group than in the placebo group. In addition, the requirement for analgesic in the esmolol group occurred after a longer period of time than in the placebo group. However, statistically there was no significant difference in this last variable between the two groups.
Go to : 
References
1. White PF, Wang B, Tang J, Wender RH, Naruse R, Sloninsky A. The effect of intraoperative use of esmolol and nicardipine on recovery after ambulatory surgery. Anesth Analg. 2003; 97:1633–1638. PMID: 14633533.

2. Yang H, Fayad A. Are beta-blockers anesthestics? Can J Anaesth. 2003; 50:627–630. PMID: 12944432.
3. Stein C, Kopf A. Anesthesia and treatment of chronic pain. In : Miller R, Eriksson L, Fleisher L, Wiener-Kronish J, Young W, editors. Miller's anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone;2010. p. 1797–1817.
4. Ono H, Ohtani N, Matoba A, Kido K, Yasui Y, Masaki E. Efficacy of intrathecal esmolol on heat-evoked responses in a postoperative pain model. Am J Ther. 2015; 22:111–116. PMID: 23411610.

5. Bhawna , Bajwa SJ, Lalitha K, Dhar P, Kumar V. Influence of esmolol on requirement of inhalational agent using entropy and assessment of its effect on immediate postoperative pain score. Indian J Anaesth. 2012; 56:535–541. PMID: 23325937.

6. Yu SK, Tait G, Karkouti K, Wijeysundera D, McCluskey S, Beattie WS. The safety of perioperative esmolol: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2011; 112:267–281. PMID: 21127279.
7. Lee SJ, Lee JN. The effect of perioperative esmolol infusion on the postoperative nausea, vomiting and pain after laparoscopic appendectomy. Korean J Anesthesiol. 2010; 59:179–184. PMID: 20877702.

8. Casalino S, Fabozzi M, Millo P, Cena A, Angellotti A, Albani A. Esmolol vs epidural anesthesia in bariatric surgery: pain control and postoperative outcome: 1AP1-1. Eur J Anaesthesiol. 2011; 28:6–7.

9. Coloma M, Chiu JW, White PF, Armbruster SC. The use of esmolol as an alternative to remifentanil during desflurane anesthesia for fast-track outpatient gynecologic laparoscopic surgery. Anesth Analg. 2001; 92:352–357. PMID: 11159231.

10. White P, Eng M. Ambulatory (out patient) anesthesia. In : Miller R, Eriksson L, Fleisher L, Wiener-Kronish J, Young W, editors. Miller's anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone;2010. p. 2426.
11. Ozturk T, Kaya H, Aran G, Aksun M, Savaci S. Postoperative beneficial effects of esmolol in treated hypertensive patients undergoing laparoscopic cholecystectomy. Br J Anaesth. 2008; 100:211–214. PMID: 18037672.

12. Celebi N, Cizmeci EA, Canbay O. Intraoperative esmolol infusion reduces postoperative analgesic consumption and anaesthetic use during septorhinoplasty: a randomized trial. Rev Bras Anestesiol. 2014; 64:343–349. PMID: 25168439.

13. Collard V, Mistraletti G, Taqi A, Asenjo JF, Feldman LS, Fried GM, et al. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesth Analg. 2007; 105:1255–1262. PMID: 17959952.

14. Stanley TH, de Lange S, Boscoe MJ, de Bruijn N. The influence of chronic preoperative propranolol therapy on cardiovascular dynamics and narcotic requirements during operation in patients with coronary artery disease. Can Anaesth Soc J. 1982; 29:319–324. PMID: 6213289.

15. Jakobsen CJ, Grabe N, Christensen B. Metoprolol decreases the amount of halothane required to induce hypotension during general anaesthesia. Br J Anaesth. 1986; 58:261–266. PMID: 3511931.

16. Chia YY, Chan MH, Ko NH, Liu K. Role of beta-blockade in anaesthesia and postoperative pain management after hysterectomy. Br J Anaesth. 2004; 93:799–805. PMID: 15377583.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download