Abstract
Background
Herpes Zoster is a disease that occurs after the virus is reactivated due to infection of the varicella virus in childhood. Risk factors are advanced age, malignant neoplasm, organ transplantation, immunosuppressive agents taking are known. The purpose of this study was to investigate the relationship between the seasonal effect and other risk factors on the incidence of herpes zoster.
Methods
The medical records of 1,105 patients admitted to the outpatient diagnosed with herpes zoster were retrospectively examined. The patients' sex, age, dermatome, onset, underlying disease, residential areas were collected.
Results
The incidence of women outnumbered men and increased for those above the age of 50. The number of occurrences of herpes zoster patients was higher in the spring and summer than in winter. Unlike men, women had the most frequent outbreaks in March. The most common occurrence of dermatome is in the thoracic region. The number of occurrence was similar on the left as the right.
Go to : 
Shingles is an infection caused by the Varicella-Zoster virus, the same virus that causes chickenpox. Although the virus lies dormant in individuals who have had chickenpox, it sometimes reactivates several years later, causing shingles. The infection is known to affect 1.5-3 individuals per 1,000 annually [123].
The known risk factors include old age, malignant neoplasm, organ transplantation, and immunosuppressive therapy. A higher incidence rate is also observed among individuals with diabetes, women, and those being treated for the human immunodeficiency virus (HIV) [4]. Dopico et al. [5] reported that there is a change in immune function according to the season. According to Miller et al. [6], chickenpox most frequently occurs in the spring and winter months, but cases of shingles do not vary by season. However, Toyama and Shiraki [7] reported that a surge of the cases was noted in the summer, which dropped in the winter.
The present study was designed based on the hypothesis that the occurrence of shingles in the rural communities of Cheonan might be affected by seasonal factors.
Go to : 
Of the individuals who visited the Soonchunhyang University Cheonan Hospital between January 1, 2012 and December 31, 2013, a total of 1,498 patients diagnosed with shingles were selected for a retrospective investigation of clinical records. Approval was obtained from the hospital's Institutional Review Board prior to the start of the investigation. Based on the following criteria, 393 patients were excluded: (1) individuals who contracted the illness before the investigation period; (2) individual exhibits no blisters, but complains of pain; (3) individual diagnosed with suspected shingles; and (4) insufficient clinical record. Data on the final 1,105 patients, pertaining to gender, age, time of occurrence, affected site (dermatome, left/right of body), underlying diseases, and place of residence, were collected. The subjects were classified into groups based on age (in increments of 5 years, beginning at 0 years), location of dermatome (trigeminal ganglion, cervical vertebra, thoracic vertebra, lumbar, or sacrum), time of occurrence (month in which rash appeared), underlying diseases (hypertension, diabetes, cerebropathy, cardiovascular diseases, respiratory diseases, renal diseases, musculoskeletal disorders, liver diseases, endocrinal diseases, cancers, exhaustion and stress, pregnancy, and miscellaneous), and by place of residence (as recorded at the time of the initial hospital visit).
Go to : 
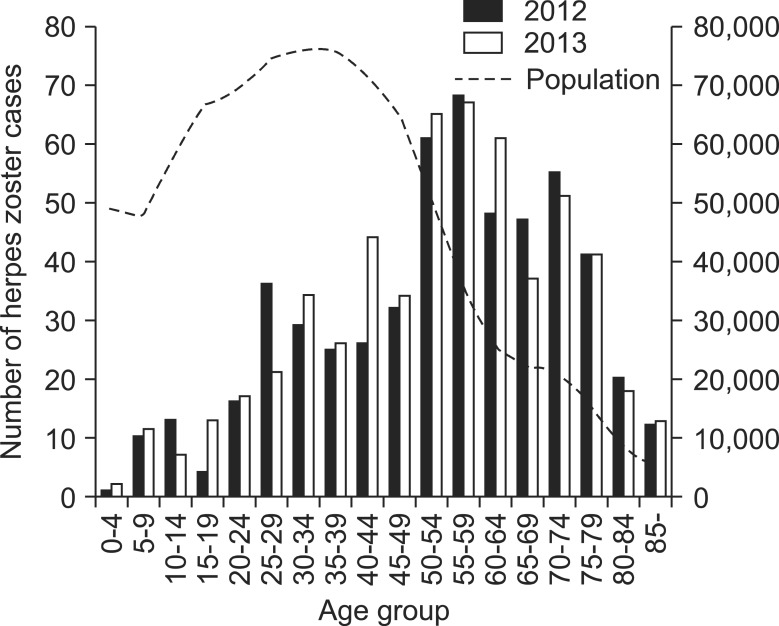
Of the 1,105 study subjects, 41.2% (456 subjects) were male and 58.8% (659 subjects) were female. The mean age was 56 years (± 19.1; range, 4-97 years). In both 2012 and 2013, a higher incidence rate was observed in the subjects 50 years of age and older, which dropped in the subjects 80 years and older (Fig. 1).
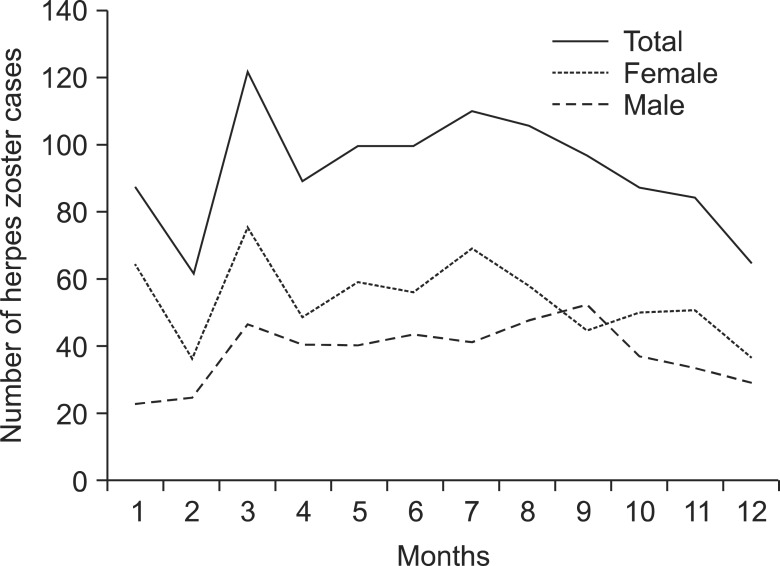
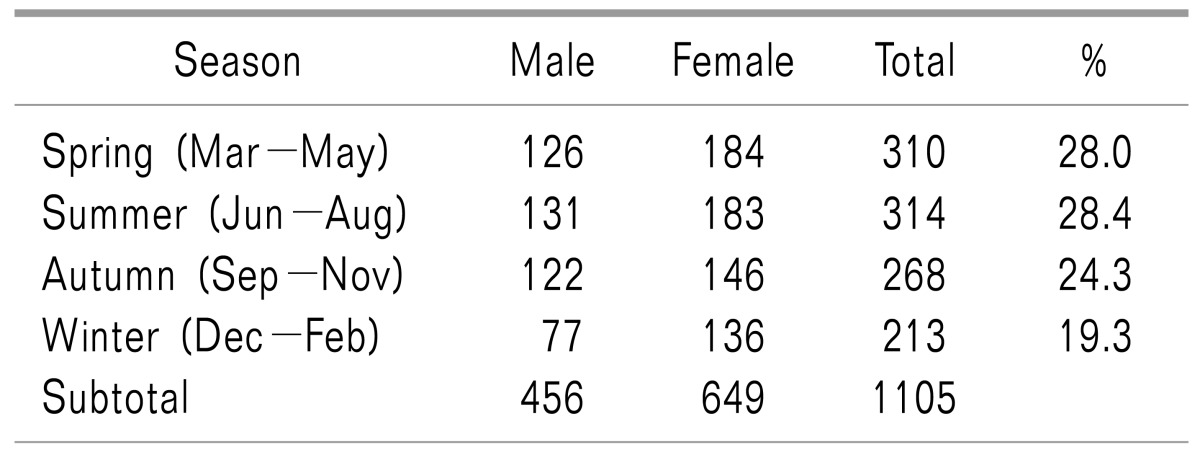
In female subjects, March showed the highest incidence rate, and February and December exhibited the lowest. In male subjects, no notable variation was found between the months of the year. The overall monthly incidence rate showed a pattern that was similar to the monthly incidence rate observed in the female subjects (Fig. 2). In terms of seasonal incidence rates, which were obtained after grouping the months by seasons, spring and summer exhibited higher incidence rates than winter (Table 1).
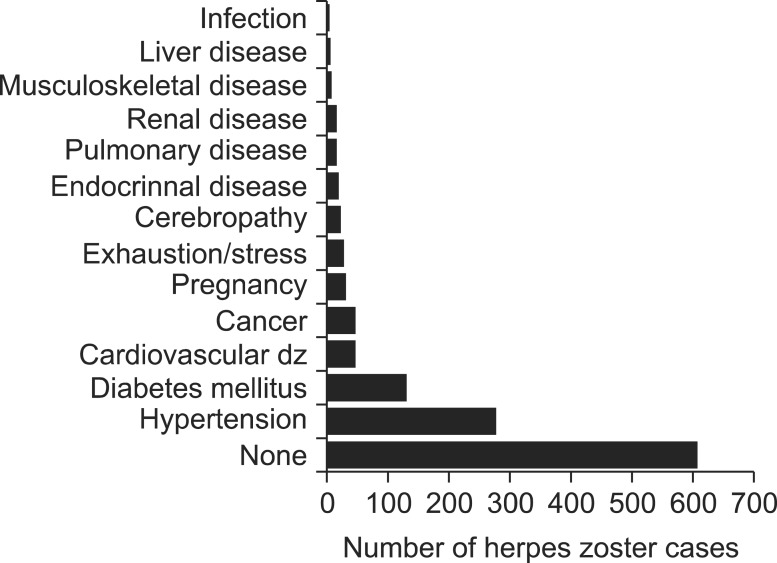
Of the 1,105 patients, 131 (11.9%) had diabetes, 46 (4.2%) had cancer, 276 (25.0%) had hypertension, 46 (4.2%) had cardiovascular diseases, 32 (2.9%) were pregnant, 29 (2.6%) reported exhaustion and stress, and 89 (8.1%) had other diseases (cerebropathy, endocrinal diseases, respiratory diseases, renal diseases, musculoskeletal disorders, liver diseases, infection, etc.). 606 (55.0%) patients reported not having any disease other than shingles (Fig. 3).
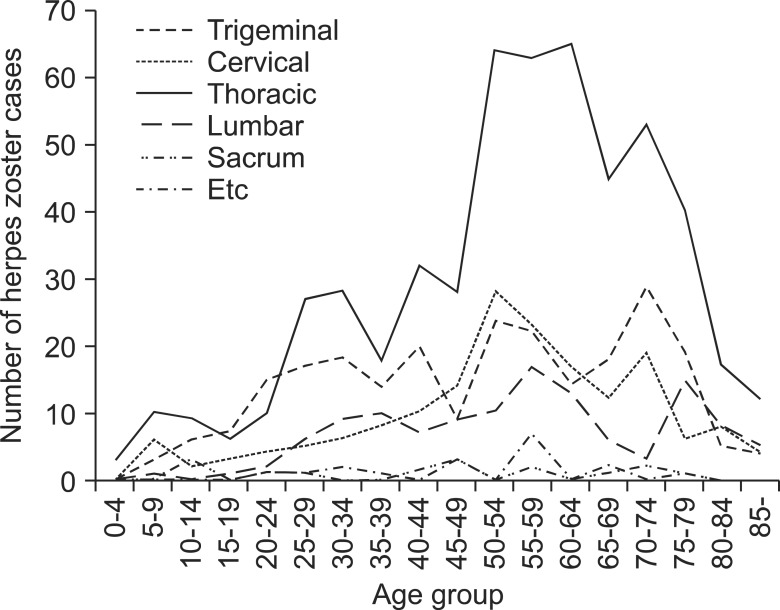
The most commonly affected site was the thoracic vertebra, reported by 530 subjects (47.9%), followed by the trigeminal ganglion reported by 237 subjects (21.4%) [ophthalmic nerve, 164 subjects (69.2%), maxillary nerve, 57 subjects (24.0%), and mandibular nerve, 16 subjects (6.8%)] (Fig. 4). No significant differences were found in the left and right trunk orientation of the rash (Left:Right = 1:1.04). Among the subjects aged 50 and older, the thoracic vertebra was the most commonly affected site (Fig. 5).
Because 76% of the study subjects resided in the neighboring area, and not enough cases were reported from elsewhere, the potential association between place of residence and season could not be traced. The number of occurrences during the study period did not appear to be affected by the trends in temperature, daily temperature range, humidity, duration of daylight, etc.
Go to : 
Through a retrospective analysis of clinical records, the present study was conducted to examine the association between shingles and seasonal factors.
It is known that older individuals and those undergoing immunotherapy for HIV infection, malignant neoplasm, organ transplantation, and steroid treatment are at a higher risk of developing shingles because of the increased likelihood of the virus reactivating. Additionally, psychological and physical stress has been reported to have an impact [8].
In general, shingles are known to affect 1.5-3 individuals per 1,000 annually [123]. However, shingles affect 15-16 individuals per 1,000 in the U.S. each year, which is attributed to the fact that most have had chickenpox as a child [8]. In Korea, shingles are known to affect 0.88-4.8 individuals per 1,000 annually [91011].
Hope-Simpson [12] reported that the rate of occurrence among individuals under the age of 10 is extremely low at 0.74 per 1,000 individuals, in contrast to 7.8 per 1,000 among individuals over the age of 60. Donahue et al. [1] reported an even higher rate of occurrence among individuals 75 years and older, at 18.4 per 1,000. In the present study, the highest incidence was observed in the 50-64 years age group, and the incidence among individuals aged 50 years and older (770 cases) was more than twice that of their younger counterparts (335 cases). In our study, the incidence was lower in the group of individuals 75 years and older. However, this appears to be due to the demographic characteristics of the study area, namely, the decline in the number of individuals in the age group (Fig. 1).
In terms of shingles and gender, Gialloreti et al. [13] reported the most significant risk factors of shingles included being female, being 55 years of age or older, and possessing a weak immune system. However, the influence of gender was not so clear [14]. A study supports the potential association between the female gender and elevated risk of shingles [15]. The study, which analyzed a total of 14,532 subjects, found a significantly higher rate of incidence among female subjects, which was attributed to differences in the immune systems between the genders. Due to the biological differences between the genders, the virus is more likely to reactivate in the female body than in the male body. The study also cited the higher exposure rate of females to children with chickenpox as one of the reasons behind the higher incidence rate [15161718]. The present study also found a higher proportion of female patients with shingles (M:F = 1:1.42). Higher rates of shingles were observed in the spring and summer months among female subjects.
Dopico et al. [5] reported that interleukin-6 (IL-6) receptor and C-reactive protein - risk biomarkers for cardiovascular, psychiatric and autoimmune diseases - have peak incidences in winter. Nelson [19] reported that exposure to short day lengths affects several parameters of the immune system. Toyama and Shiraki [7] analyzed a total of 48,388 shingles cases and found that occurrence of shingles increased in the summer and decreased in the winter. The study also reported a higher rate of occurrence among females and individuals in the 50-70 years age group. On the other hand, Hope-Simpson [12] reported high rates of occurrence in the summer and fall. Zak-Prelich et al. [20] reported that exposure to UV rays increased the rates of shingles affecting the face among the male subjects. Other studies, however, found no correlation between shingles and seasons [22122]. The present study found that shingles had a higher rate of occurrence in the spring and summer months among female subjects than in male subjects.
In terms of the affected site and time of occurrence, no correlation was found, with the exception of the thoracic vertebrae, which showed a greater incidence in the spring and summer, similar to the overall trend. Similar incidence rates were observed in the left and right sides of the body throughout the seasons.
The places of residence of the study subjects were mostly in Cheonan and neighboring areas, such as Asan, and the incidence during the study period was not affected by weather conditions such as temperature, daily temperature range, humidity, or duration of daylight.
Based on these findings, it is plausible that immunization in the winter months may reduce the incidence of shingles in Cheonan and neighboring areas.
This study has several limitations. Since the study subjects were limited to the patients diagnosed with shingles at the hospital, the area incidence rate could not be identified. As a retrospective study, only data regarding the places of residence could be incorporated, and therefore, the findings do not reflect the potential influence of socioeconomic factors on shingles, such as occupation and residential environment. Finally, data collection regarding underlying diseases may have been inadequate as it relied only on the existing clinical record. For a more detailed investigation of the influence of seasons on the occurrence of shingles, changes in the natural, living, and occupational environment need to be examined.
In the present study, a higher incidence rate was observed in females than in males, and the rate was higher in the spring and summer. Based on our findings, shingles immunization in the winter months could be beneficial for residents in the neighboring area.
Go to : 
References
1. Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995; 155:1605–1609. PMID: 7618983.

2. Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982; 61:310–316. PMID: 6981045.

3. Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005; 20:748–753. PMID: 16050886.

4. Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013; 67:463–469. PMID: 23872209.

5. Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ, et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun. 2015; 6:7000. PMID: 25965853.

6. Miller E, Marshall R, Vurdien J. Epidemiology, outcome and control of varicella-zoster infection. Rev Med Microbiol. 1993; 4:222–230.

7. Toyama N, Shiraki K. Society of the Miyazaki Prefecture Dermatologists. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol. 2009; 81:2053–2058. PMID: 19856466.

8. Wharton M. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am. 1996; 10:571–581. PMID: 8856352.

9. Shin DY, Koo DW. Statistical analysis of herpes zoster in Chuncheon and the northern Kangwon province (1994-1996). Korean J Dermatol. 1998; 36:422–429.
10. Park SY, Kim JY, Kim CD, Kim CW, Lee KS. A clinical study on herpes zoster during the last 10-year-period (1994-2003). Korean J Dermatol. 2004; 42:1531–1535.
11. Kim SY, Cho BH, Kim JH. A 5-year clinical study on herpes zoster: 1990-1994. Korean J Dermatol. 1997; 35:266–272.
13. Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010; 10:230. PMID: 20682044.

14. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004; 4:26–33. PMID: 14720565.

15. Fleming DM, Cross KW, Cobb WA, Chapman RS. Gender difference in the incidence of shingles. Epidemiol Infect. 2004; 132:1–5. PMID: 14979582.

16. Law B, Fitzsimon C, Ford-Jones L, McCormick J, Rivière M. Cost of chickenpox in Canada: part II. Cost of complicated cases and total economic impact. The Immunization Monitoring Program-Active (IMPACT). Pediatrics. 1999; 104:7–14. PMID: 10390253.

17. Somekh E, Dalal I, Shohat T, Ginsberg GM, Romano O. The burden of uncomplicated cases of chickenpox in Israel. J Infect. 2002; 45:233–236. PMID: 12423610.

18. Yu SJ, Lee SM, Chung KD, Youn EK, Yoon KJ. Herpes zoster in healthy child: a case report. Korean J Pain. 2008; 21:71–73.

19. Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004; 25:187–192. PMID: 15039045.

20. Zak-Prelich M, Borkowski JL, Alexander F, Norval M. The role of solar ultraviolet irradiation in zoster. Epidemiol Infect. 2002; 129:593–597. PMID: 12558343.

21. Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001; 127:305–314. PMID: 11693508.

22. Chant KG, Sullivan EA, Burgess MA, Ferson MJ, Forrest JM, Baird LM, et al. Varicella-zoster virus infection in Australia. Aust N Z J Public Health. 1998; 22:413–418. PMID: 9659764.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download