Abstract
We are reporting a rare case of a delayed hypersensitivity reaction caused by hyaluronidase allergy following a lumbar transforaminal epidural block. Using an intradermal skin test, we have provided evidence that the systemic allergic reaction resulted from hypersensitivity to hyaluronidase. To our knowledge, this is a rare case of a delayed hypersensitivity reaction to epidural hyaluronidase, comprised of an initial exposure to hyaluronidase with no subsequent allergic response in prior block followed by a subsequent delayed reaction to hyaluronidase during a second epidural block.
Go to : 
Hyaluronidase is an enzyme with spreading activity that hydrolyzes hyaluronic acid, a key element of connective tissue [1,2]. In general, hyaluronidase reduces tissue swelling caused by trauma, as well as fibrosis from fibrin deposits and chronic inflammation [3]. By decreasing the hyaluronic acid viscosity, it increases tissue permeability, improving the spread and efficacy of local anesthetics. For these reasons, hyaluronidase has been gaining popularity among pain physicians. Despite the widespread use of hyaluronidase, only a few allergic reactions related to the enzyme have been reported. Most of the reports have described allergic reactions following peribulbar injections for cataract surgery [1,4,5,6,7]. We report a case of a delayed hypersensitivity reaction caused by hyaluronidase allergy following a lumbar transforaminal epidural block. Using an intradermal skin test, we have provided evidence that the systemic allergic reaction resulted from hypersensitivity to hyaluronidase.
A 46-year-old woman suffering from severe back pain caused by a ruptured herniated neucleosus pulposus (HNP) at the level of the L1-2 intervertebral disc underwent a transforaminal epidural block. We used 5 ml of 0.5% ropivacaine, 2 mg of dexamethasone, 1,500 IU of hyaluronidase (H-lase®, Gunil, Seoul, Korea, powder type), and 1 ml of contrast media (TELEBRIX®, Guerbet, France). The patient had no history of drug allergy and had previously received an uneventful inter-laminar epidural block, which had been performed with the same mixture of drugs. After the injection, the patient remained stable for a while. However, several minutes later, she complained of heat and nausea, chest tightness, a generalized rash and angioedema around the eyelids and the lips. The heart rate (HR) of the patient was between 130 to 140 bpm, although there was no hypotensive crisis. Under close observation of her vital signs, she was treated with dexamethasone and antihistamine intravenously. After several hours, the symptoms diminished.
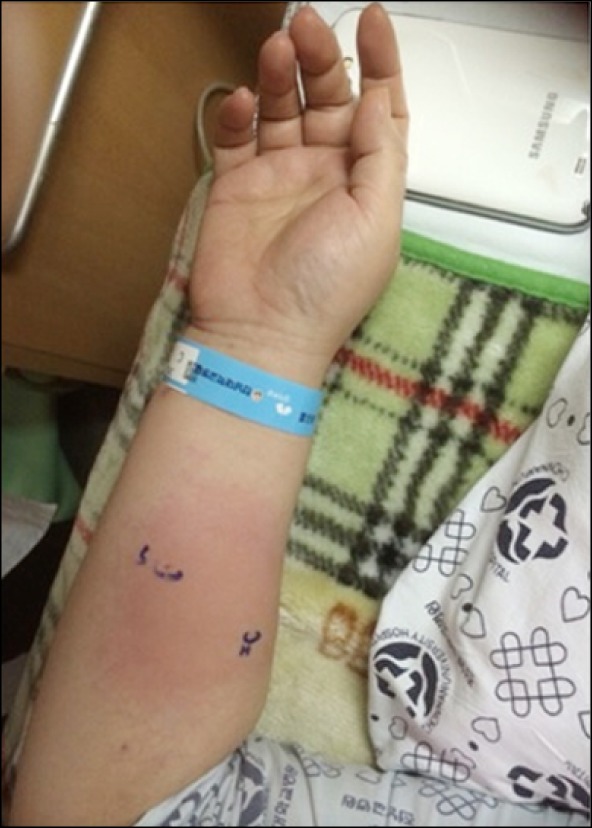
About 4 months later, the patient's back pain had returned, and we conducted a second transforaminal epidural block in the same region. Unfortunately, we accidentally used the same regimen containing hyaluronidase. Up to 1 hour after the procedure, the patient did not complain of any particular symptoms, and we determined that she could be discharged. After discharge, however, as she waited for medical treatment from another department, she began to run a fever and complained of nausea, vomiting, and chest pain. We injected dexamethasone 5 mg intramuscularly and transferred her to the PACU for management of her vital signs. At the PACU, her blood pressure was 110/60 mmHg, her heart rate was 150 bpm, and her peripheral oxygen saturation (SpO2) fluctuated. Moreover, she presented generalized rash and edema all around her body. We loaded crystalloids (Hartmann solution), and injected dexamethasone 5 mg intravenously. Three hours later, the symptoms decreased and her vital signs became more stable (BP 120/70 mmHg, HR 90-100 bpm, SpO2 94-95%). After full recovery of her general condition, we performed an intradermal skin test to identify the cause of the allergic reaction. A positive result was found for hyaluronidase (15 IU/ml) with a persistent wheal of more than 0.8 cm (Fig. 1) [8]. The skin test showed no positive results for 0.5% ropivacaine, contrast media, 2% lidocaine, or 5% povidone-iodine.
Go to : 
Allergic reactions to hyaluronidase are uncommon. The estimated incidence in an ophthalmic surgery department was 0.1% [6]. Reports of allergic reactions to hyaluronidase have been increasing over the last decade, as the latter is being used more commonly in various medical fields. These days, epidural injections of hyaluronidase are frequently performed in pain clinics [9,10].
The initial manifestations of a hyaluronidase allergy are mainly influenced by the route of administration and the dosage of the drug. In general, when the dose ranges from 100 to 150 IU and is injected at a local site, allergic reactions to hyaluronidase are confined to localized responses (edema, urticaria) in the injection area without generalized symptoms [4]. On the other hand, when the administrated dose ranges from 1,500 to 200,000 IU and is injected intravenously, a regimen commonly used as an additive to chemotherapeutic drugs in oncology, most patients present more generalized symptoms, such as anaphylactic shock, urticaria, or dyspnea [2,11]. Szepfalusi et al. reported that five of sixteen pediatric cancer patients showed immediate generalized allergic reactions following intravenous injection of a hyaluronidase and chemotherapeutic agent mixture [2].
In this case, the first injection of hyaluronidase did not induce an allergic response, but repeated use induced sensitization and a generalized allergic response appeared. A similar case of sensitization to a secondary use of hyaluronidase has previously been reported [12]. In that case, a caudal block with hyaluronidase was undertaken, and the patient showed an immediate allergic reaction following the administration of hyaluronidase. In our case, on the other hand, the patient remained stable without complaining of any allergic symptoms up to several minutes after the procedure.
Systemic reactions in these cases are regarded as a result of systemic absorption of the drug. However, no obvious intravascular injections have been identified by imaging the spread of the contrast media. Considering the rich vascularity of the epidural spaces and capillary dilations induced by hyaluronidase, the delayed onset of the allergic reaction can be explained by the slow transition of the drugs from the epidural space into the systemic circulation. Thus, it seems that systemic allergic reactions can occur in patients with hyaluronidase hypersensitivity, even if the epidural block is performed properly.
This report was limited by the lack of immunologic assay conducted with blood samples obtained at the time of the attack. The proposed mechanism for hyaluronoidase hypersensitivity is a combination of type 1 hypersensitivity (IgE mediated, immediate) and type 4 hypersensitivity (T-cell mediated, delayed) [1,4]. Considering the onset time and the duration of the allergic symptoms, type I hypersensitivity seems more likely to have been the mechanism at work in the present case. On the other hand, the sensitization to the initial exposure of hyaluronidase and the allergic reactions to its secondary use are more characteristic of type 4 hypersensitivity. In this case, measurement of the elevated patient's serum-specific IgE antibody to hyaluronidase and a T-cell count analysis after the skin patch test could be helpful to identify the mechanism of hypersensitivity. Regarding the prophylaxis aspect, however, an intradermal skin test can be a useful method to predict allergies. In many reports, most of the intradermal skin tests performed on patients with hyaluronidase hypersensitivity were positive [1,6]. Therefore, skin tests are necessary to prevent systemic allergic reactions, particularlly in patients who are supposed to be administrated a high dose of hyaluronidase.
In conclusion, it is necessary to suspect the hyaluronadase hypersensitivity in patients showing post-procedural allergic reactions, even if previous epidural uses of hyaluronidase were uneventful. Additionally, special caution needs to be taken to avoid life-threatening allergic reactions if the patient had a previous allergic reaction to hyaluronidase.
Go to : 
References
1. Delaere L, Zeyen T, Foets B, Van Calster J, Stalmans I. Allergic reaction to hyaluronidase after retrobulbar anaesthesia: a case series and review. Int Ophthalmol. 2009; 29:521–528. PMID: 18784901.

2. Szépfalusi Z, Nentwich I, Dobner M, Pillwein K, Urbanek R. IgE-mediated allergic reaction to hyaluronidase in paediatric oncological patients. Eur J Pediatr. 1997; 156:199–203. PMID: 9083759.

3. Geurts JW, Kallewaard JW, Richardson J, Groen GJ. Targeted methylprednisolone acetate/hyaluronidase/clonidine injection after diagnostic epiduroscopy for chronic sciatica: a prospective, 1-year follow-up study. Reg Anesth Pain Med. 2002; 27:343–352. PMID: 12132057.

4. Kempeneers A, Dralands L, Ceuppens J. Hyaluronidase induced orbital pseudotumor as complication of retrobulbar anesthesia. Bull Soc Belge Ophtalmol. 1992; 243:159–166. PMID: 1302146.
5. Kirby B, Butt A, Morrison AM, Beck MH. Type I allergic reaction to hyaluronidase during ophthalmic surgery. Contact Dermatitis. 2001; 44:52. PMID: 11156027.

6. Leibovitch I, Tamblyn D, Casson R, Selva D. Allergic reaction to hyaluronidase: a rare cause of orbital inflammation after cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2006; 244:944–949. PMID: 16362314.

7. Minning CA Jr. Hyaluronidase allergy simulating expulsive choroidal hemorrhage. Arch Ophthalmol. 1994; 112:585–586. PMID: 8185511.

8. Feighery C, McCoy EP, Johnston PB, Armstrong DK. Delayed hypersensitivity to hyaluronidase (Hyalase) used during cataract surgery. Contact Dermatitis. 2007; 57:343. PMID: 17937752.

9. Avellanal M, Diaz-Reganon G. Interlaminar approach for epiduroscopy in patients with failed back surgery syndrome. Br J Anaesth. 2008; 101:244–249. PMID: 18552347.

10. Schulze C, Bittorf T, Walzel H, Kundt G, Bader R, Mittelmeier W. Experimental evaluation of hyaluronidase activity in combination with specific drugs applied in clinical techniques of interventional pain management and local anaesthesia. Pain Physician. 2008; 11:877–883. PMID: 19057633.
11. Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007; 80:1921–1943. PMID: 17408700.

12. Kim TW, Lee JH, Yoon KB, Yoon DM. Allergic reactions to hyaluronidase in pain management -A report of three cases-. Korean J Anesthesiol. 2011; 60:57–59. PMID: 21359084.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download