INTRODUCTION
Neuroendocrine tumors (NETs) are low-grade malignancies arising from neuroendocrine cells. NET rarely occurs primarily in the gastrointestinal tract (54.5%) and bronchus (30.1%).
1 Primary hepatic NETs (PHNETs) are extremely rare, but the liver is the most common organ affected by metastatic NET. The most frequently reported symptom of PHNET is non-specific abdominal pain (33%).
2,3 PHNET is difficult to differentiate from other liver tumors, such as hepatocellular carcinoma (HCC) or cholangiocarcinoma, based on radiological findings. Hence, it is diagnosed based on immunohistochemical analysis after surgery.
This paper reports a case of PHNET, in which the preoperative radiologic diagnosis was HCC, but the tumor was confirmed postoperatively as PHNET by immunohistochemistry. The present case is meaningful because it was the youngest diagnosed thus far.
Go to :

CASE REPORT
A 22-year-old male presented with intermittent abdominal pain lasting three months. The patient showed normal vital signs. A physical examination showed that the abdomen was generally soft, but no palpable mass was noted. The blood tests showed normal values for AFP and PIVKA II (protein induced by vitamin K absence or antagonists-II) as tumor markers and negative hepatitis B and C serology. An esophagogastroduodenoscopy revealed an ulcer in the duodenal bulb, but otherwise normal findings. The duodenal lesion was pathologically confirmed as a chronic ulcer. Ultrasonography (US) revealed a 5×4 cm-sized mixed echogenic mass with an anechoic area in the right hepatic lobe was observed, which was considered to be hemangioma or other liver tumor (
Fig. 1). Arterial phase abdominal CT showed a 5.0 cm-sized heterogeneously enhancing mass in the S6 of the liver with a partial enhancing pattern in the portal phase (
Fig. 2). In contrast, MRI revealed a 5.0 cm-sized well-defined solid mass with a cystic component. The solid mass showed arterial hyperenhancement and a delayed washout pattern in the portal phase, strongly indicating HCC (
Fig. 3).
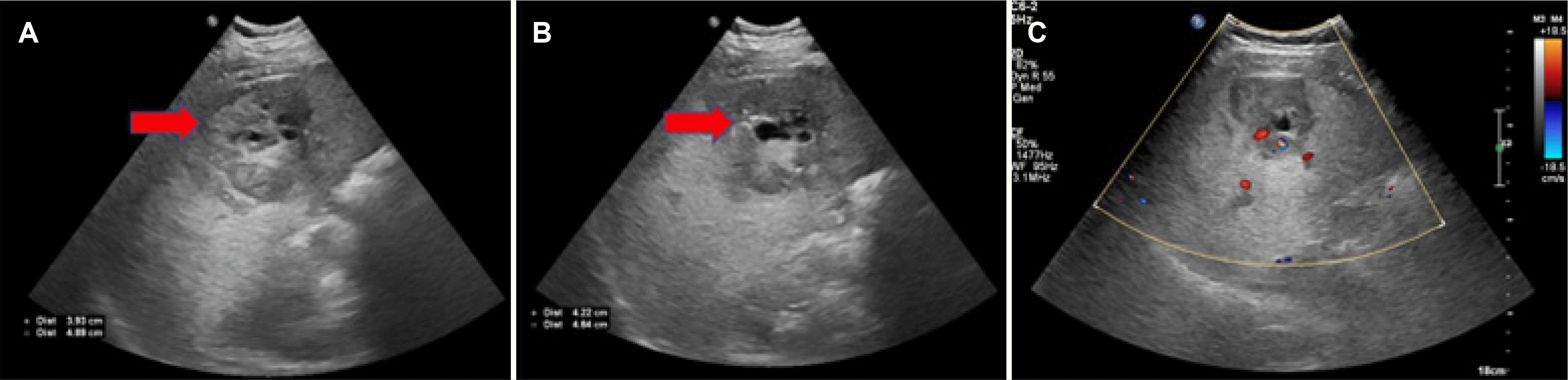 | Fig. 1(A-C) Abdominal ultrasonography and color Doppler image showed 5.0×4.0 cm mixed echoic mass in right hepatic lobe (arrows of A, B). 
|
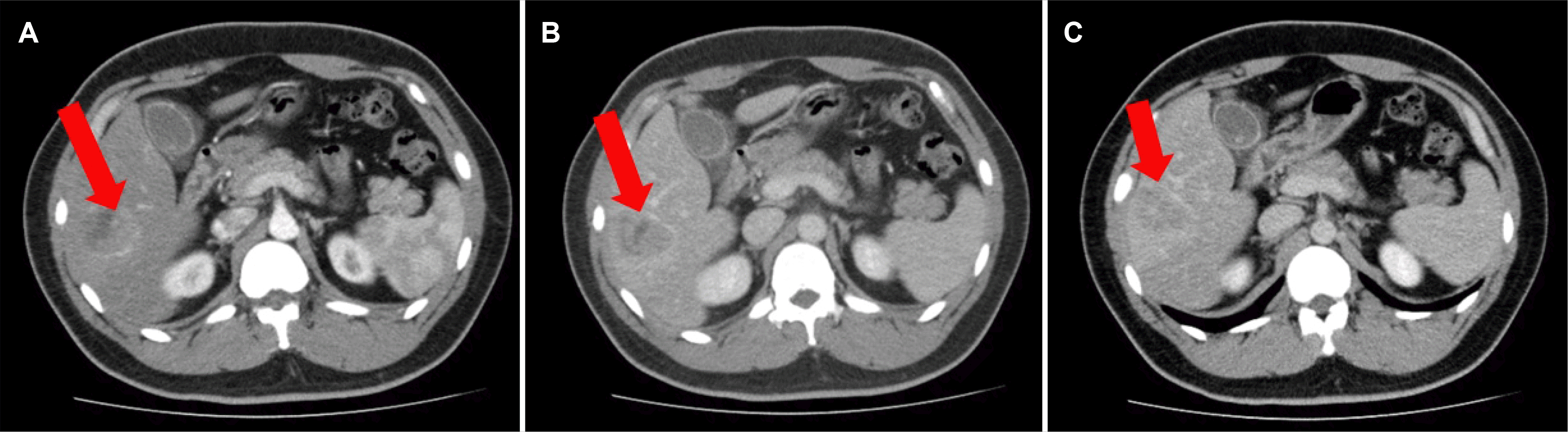 | Fig. 2(A-C) Abdominal computed tomography showed a 5.1 cm enhancing mass in S6 of the liver in the arterial phase, partial enhancing in the portal phase, and a washout pattern in the delayed phase (arrow in A, arterial phase; B, portal phase; C, delayed phase). 
|
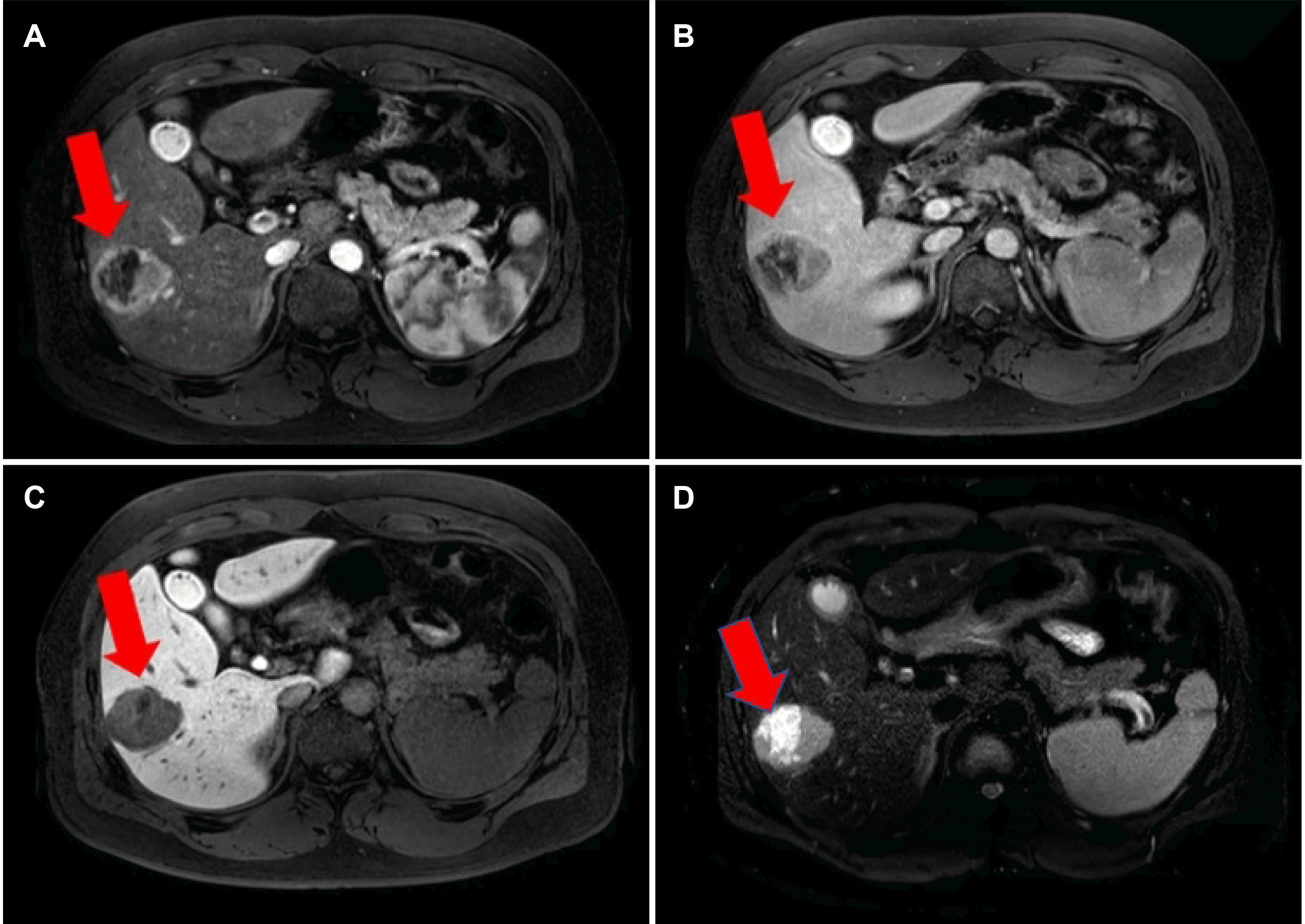 | Fig. 3(A) Abdominal magnetic resonance imaging showed the lesion demonstrating peripheral enhancement on the arterial phase (arrow), (B) contrast washout with peripheral rim enhancement on the portal (arrow), (C) 20-min delayed phase (arrow), and (D) cystic component (T2WI; arrow). 
|
Laparoscopic right hepatectomy was performed based on a preoperative diagnosis of HCC of the right liver lobe. A whitish mass was observed on the liver surface in the surgical field. Pathologically, a 6.6×4.2 cm-sized yellow-to-brown solid mass was found in the S6 parenchyma. No tumor cells were present at the surgical margins (
Fig. 4). Microscopy of the mass showed tumor cells with abundant eosinophilic cytoplasm and uniform round nuclei. Immunohistochemically, the tumor cells were positive for epithelial markers, such as cytokeratin, and neuroendocrine markers, including CD56, synaptophysin, chromogranin A (CgA), and neuron-specific enolase, with a Ki-67 labeling index of 1-2%, and a mitotic rate of 1/10 high power field (HPF). A final diagnosis of NET, G1 was rendered (
Fig. 5).
18F-fluorodeoxyglucose (FDG)-PET/CT was performed postoperatively to identify the extrahepatic lesions. No lesions outside the liver were found. The patient recovered uneventfully.
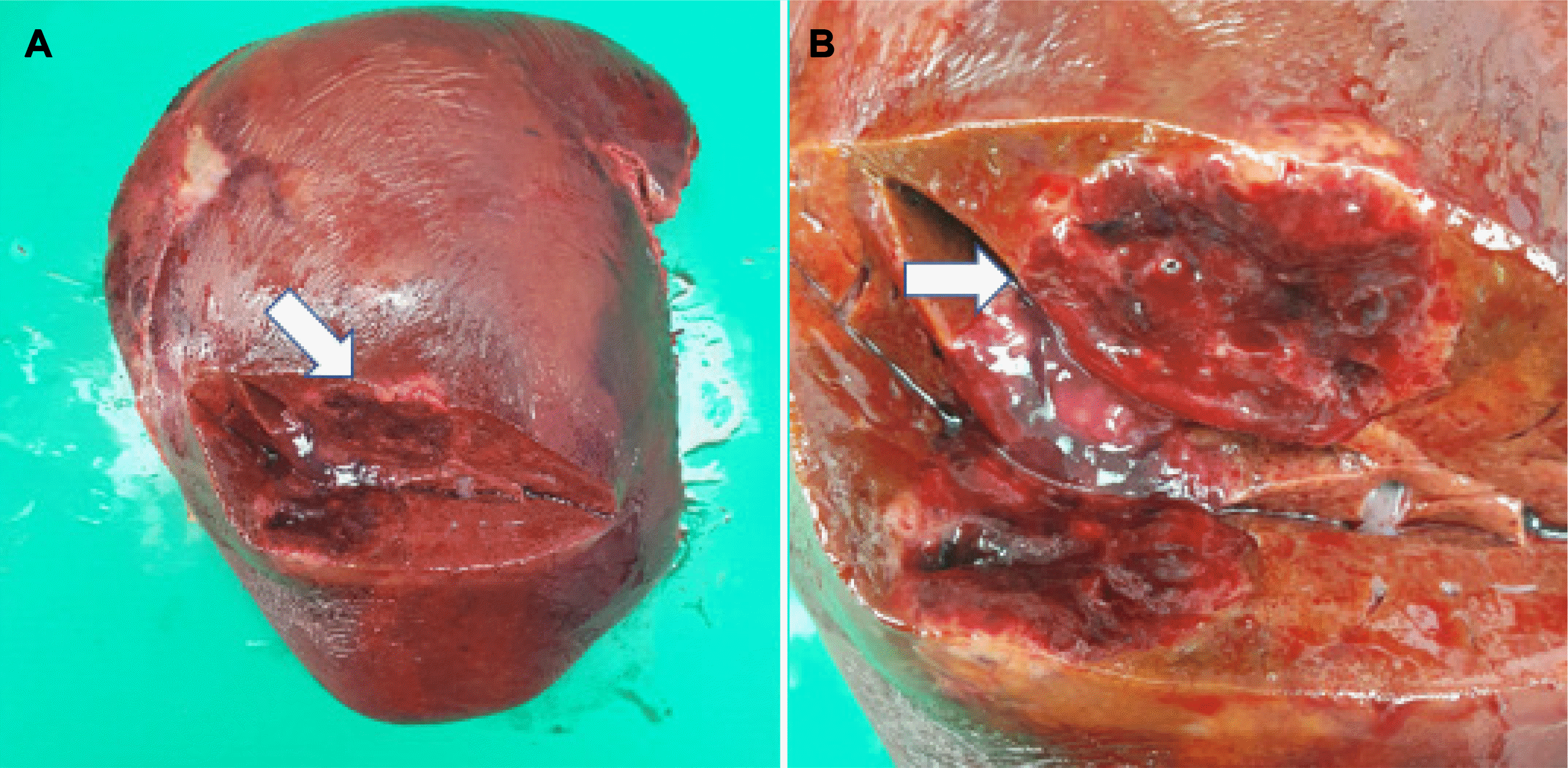 | Fig. 4(A, B) Surgical specimen of the liver revealed a 6.6×4.2 cm yellow-to-brown solid mass in S6 (arrows). 
|
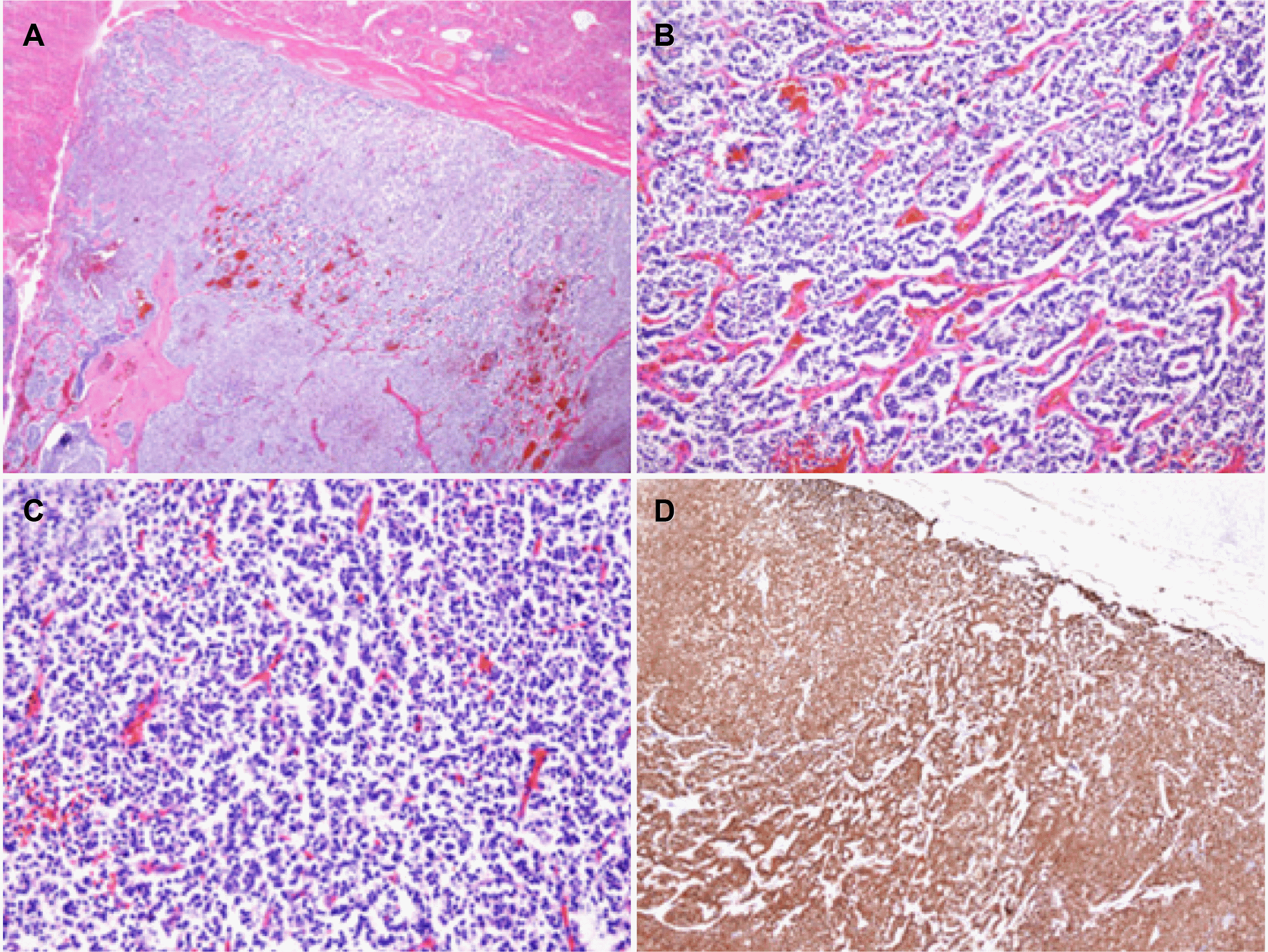 | Fig. 5Microscopy findings of the liver mass showed (A) a well-demarcated tumor (hematoxylin and eosin stain [H&E], ×20), and (B) diffuse and cord-like growth pattern of tumor cells (H&E, ×40), and (C) uniform round to ovoid tumor cells (H&E, ×100), and (D) in immunohistochemical staining, the tumor cells were positive for chromogranin (×20). 
|
Go to :

DISCUSSION
According to the 2019 World Health Organization (WHO) neuroendocrine neoplasms classification, well differentiated are classified as NET and poorly differentiated as neuroendocrine carcinoma. Among them, NETs are classified into grades one-three according to the mitotic rate. Grades 1, 2, and 3 are classified as low, intermediate, and high, respectively. NET rarely occurs primarily in the gastrointestinal tract (up to 50%) and bronchus (up to 30%).
1 Although PHNET is extremely rare (up to 0.3%), the liver is the most common organ affected by metastatic NET (32-75%).
3 Therefore, the possibility of metastatic NET should be considered in cases where NET is found in the liver. An octreotide scan has a high sensitivity of 85-90% for metastatic NET to the liver but relatively low sensitivity for PHNET.
4 On the other hand, an octreotide scan was not conducted in the present case because the radiologic findings strongly suggested HCC. Furthermore, both esophagogastroduodenoscopy and colonoscopy might be conducted to locate a GI tract lesion.
2 A preoperative CT scan showed arterial enhancement and portal phase partial enhancement followed by washout in the delayed phase, which is similar to HCC.
5 Preoperative MRI showed a solid mass with early enhancement and a delayed washout pattern and presented a cystic portion of hypo-enhancement in T1-weighted enhancement in the T2-weighted image in the solid mass. In up to 40% of HCC patients, some hypervascular lesions with rapid growth may show cystic or hemorrhagic changes on the MRI findings.
6,7 Therefore, it is difficult to diagnose PHNET preoperatively using a radiographic examination alone. Although preoperative blood tests and tumor marker tests show normal results, different levels of CgA and 5-hydroxyindole acetic acid (5-HIAA) can help diagnose NET. While 5-HIAA has a low sensitivity for PHNET,
8 CgA has a sensitivity of 85-100% and a specificity of 85-95% for the tumor.
Therefore, CgA can be a useful marker for diagnosing PHNET.
9 There are no recommendations for the treatment of PHNET.
2,8,10 According to previous reviews of hepatectomy for PHNET, the 5-year survival rate after surgery was 75-80% with a recurrence rate of 18%.
11 Other therapeutic options include liver transplantation, trans-arterial chemoembolization (TACE), and radiofrequency ablation (RFA). TACE is effective in PHNET with intrahepatic metastasis, and RFA can be useful for unresectable PHNET.
12,13 The prognosis of PHNET varies according to the NET grade based on the WHO classification. In 2016, Park et al.
2 reported that grade 3 NETs have poor prognoses. The lower cellular proliferation grades (G1/G2) were demonstrated as a favorable prognostic factor for PHNET.
9,14 In addition, previous reports showed a tendency that the older patients presented with a higher tumor grade. This case was diagnosed at a young age and had a low tumor grade which followed the tendency. A literature review revealed 19 domestic cases where surgical approaches were performed, including the present case, from January 1997 to February 2021 (
Table 1).
15-20 All were diagnosed in their thirties or older. The radiologic findings of these cases showed an arterial phase enhanced solid mass with or without cystic portion. Similar to the present case, another two cases were initially diagnosed with HCC, and TACE was then performed before liver resection.
15 Ki-67 proliferative index was the only significant risk factors for tumor recurrence.
15 Recurrent cases were positive surgical tumor margin (R1 resection) grade 3.
15 No recurrence occurred in G1.
15 The present case was diagnosed with HCC from the radiology findings, but PHNET was diagnosed after a surgical resection. This patient is the youngest case. The Ki-67 proliferative index was 2%, and the mitotic rate was 1/10 HPF, which indicated NET, G1. In addition, the probability of recurrence in the future is expected to be low because an R0 resection was performed.
Table 1
Clinical and Tumor Characteristics of the Primary Hepatic Neuroendocrine Tumor in the Patient Group Who Underwent Surgical Approach in Korea (from January 1997 to February 2021)
|
No. |
Sex |
Age |
Clinical features |
Tumor No. |
Image finding |
Tumor size (cm) |
Operation |
Resecti on type |
Tumor grade |
Timing of recurrence (month) |
|
1 (Jung et al.15 [2019]) |
M |
48 |
Abdominal pain |
1 |
N/D |
12.0 |
RH |
R0 |
G1 |
- |
|
2 |
F |
45 |
Abdominal pain |
1 |
Heterogenous arterial enhancement and washout mass |
4.3 |
LMS |
R0 |
G1 |
- |
|
3 |
F |
37 |
Abdominal pain |
1 |
N/D |
4.0 |
LH+BDR+PVT |
R1 |
G3 |
13 |
|
4 |
M |
55 |
Incidental |
1 |
N/D |
4.2 |
RH |
R0 |
G2 |
- |
|
5 |
F |
42 |
Incidental |
1 |
N/D |
3.2 |
LMS+S1+BDR |
R0 |
G1 |
- |
|
6 |
F |
64 |
Abdominal pain |
1 |
N/D |
15.0 |
RH |
R0 |
G3 |
47 |
|
7 |
M |
62 |
Abdominal pain |
1 |
Heterogenous arterial enhancement and washout mass |
8.0 |
CBS |
R0 |
G3 |
1 |
|
8 |
M |
50 |
Abdominal pain |
1 |
N/D |
18.0 |
LTS |
R1 |
G3 |
2 |
|
9 |
F |
48 |
Incidental |
1 |
N/D |
3.0 |
RPS |
R0 |
G2 |
23 |
|
10 |
M |
26 |
Abdominal pain |
1 |
Hypervascular mass and central necrosis |
29.0 |
LH |
R0 |
G2 |
5 |
|
11 |
M |
69 |
Chest discomfort |
1 |
N/D |
4.2 |
RPS |
R0 |
N/D |
- |
|
12 |
M |
48 |
Abdominal discomfort |
1 |
Well demarcated mass at the arterial phase |
4.6 |
LTS |
R0 |
G1 |
- |
|
13 |
M |
70 |
Abdominal discomfort |
1 |
N/D |
15.0 |
CBS |
R1 |
G3 |
1 |
|
Oh et al.16 (1998) |
M |
48 |
Epigastric pain |
1 |
Arterial phase enhanced solid mass and cystic lesion |
13.0 |
RH |
R0 |
N/D |
N/D |
|
Kim et al.18 (2021) |
F |
51 |
Abdominal discomfort |
1 |
Multi-locular cystic mass |
14.0 |
LH |
R0 |
G2 |
N/D |
|
Lee et al.20 (2015) |
M |
67 |
Lt. hemiparesis |
1+brain metastasis |
Rim-enhancing mass with central necrosis |
10.0 |
RH |
R0 |
G3 |
N/D |
|
Kim et al.17 (2013) |
F |
67 |
Nausea |
1 |
Heterogenous arterial enhancement and washout mass |
10.0 |
CH |
R0 |
G3 |
N/D |
|
Lee et al.19 (2006) |
F |
75 |
Fever RUQ pain |
1 |
Multi-locular cystic mass |
20.0 |
Aspiration No resection |
- |
N/D |
N/D |
|
Our case |
M |
22 |
Epigastric pain |
1 |
Solid massand cystic component |
6.0 |
RH |
R0 |
G1 |
- |

There were some limitations in the present case. The liver is the most common organ affected by metastatic NET, so the first step is to find the other primary lesion when NET is diagnosed in the liver. GI tract NETs should have been excluded by esophagogastroduodenoscopy and colonoscopy. In addition, FDG-PET/CT was performed to check for metastases after surgery. An octreotide scan would be preferable for finding the primary lesion of NET than FDG-PET/CT.
This study experienced a case of PHNET that occurred at a young age. The case was misdiagnosed preoperatively as HCC because of the enhancing pattern radiologically. In case of a young age and no risk factor of HCC, it is important to consider PHNET as a possible diagnosis of hypervascular tumors.
Go to :








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download