Abstract
Background
Caudal epidural injections have been commonly performed in patients with low back pain and radiculopathy. Although caudal injection has generally been accepted as a safe procedure, serious complications such as inadvertent intravascular injection and dural puncture can occur. The present prospective study was designed to investigate the influence of the depth of the inserted needle on the success rate of caudal epidural blocks.
Methods
A total of 49 adults scheduled to receive caudal epidural injections were randomly divided into 2 groups: Group 1 to receive the caudal injection through a conventional method, i.e., caudal injection after advancement of the needle 1 cm into the sacral canal (n = 25), and Group 2 to receive the injection through a new method, i.e., injection right after penetrating the sacrococcygeal ligament (n = 24). Ultrasound was used to identify the sacral hiatus and to achieve accurate needle placement according to the allocated groups. Contrast dyed fluoroscopy was obtained to evaluate the epidural spread of injected materials and to monitor the possible complications.
Go to : 
Epidural injections are classified as transforaminal, interlaminar and caudal injection according to the routes of entry. Caudal epidural steroid injections have been commonly used as diagnostic or therapeutic tool in a variety of lumbosacral-originating spinal pain, and particularly it can be applied to patients with complicating lumbar epidural access conditions, such as postlaminectomy syndrome. In the conventional caudal epidural technique, the needle is inserted at 45-60° to the skin surface until the sacrococcygeal ligament is penetrated with a characteristic "pop", and the needle is then lowered to 15-30° and advanced 1 cm further into the sacral canal. It is considered a relatively easy technique in the interventional pain management field, and is also known to present a lower risk of accidental dural puncture than other epidural techniques [1].
However, conventional caudal epidural injections present a potential risk of penetration of the epidural venous plexus or dura. According to our literature review, the incidence of accidental intravascular injections as confirmed by contrast enhanced fluoroscopy ranged between 11-42% in patients who had received caudal epidural injections [2,3,4,5]. Moreover, there have been a few reports of inadvertent dural puncture during the caudal approach caused by an abnormally low termination of the dural sac in the sacral canal [6]. To our knowledge, accidental intravascular and intrathecal drug injections can cause systemic toxicity of the local anesthetics and total spinal anesthesia, if they are not detected by the physicians beforehand.
In this context, we hypothesized that a new caudal injection technique, which the needle only penetrates the sacrococcygeal ligament without being inserted into the sacral canal, might represent a safe alternative, with a lower incidence of intravascular and intrathecal injections than the conventional technique. The present study prospectively investigated the influence of the depth of the needle insertion in ultrasound- guided caudal epidural injection, and evaluated the feasibility of the new caudal injection method.
Go to : 
This study was approved by the institutional review board of the hospital. After obtaining informed consent from the participants, 50 patients, aged 19-80 years, who were scheduled to receive caudal epidural injections for low back pain with or without radiculopathy in our pain clinic were enrolled in this prospective study. Patients with a history of lumbosacral spine surgery, allergies to local anesthetics or contrast dye, coagulation abnormalities, or suspicions of infections in the coccygeal area were excluded from the study. Using a computerized random number generator, the patients were randomly allocated to one of two groups, a conventional method group (Group 1), receiving the injection after further needle advancement of 1 cm into the sacral canal following penetration of the sacrococcygeal ligament, or a new method group (Group 2), receiving the injection immediately after penetration of the sacrococcygeal ligament. Patients were given consent with full knowledge of the risks involved, including radiation exposure, possible consequences, and the alternatives.
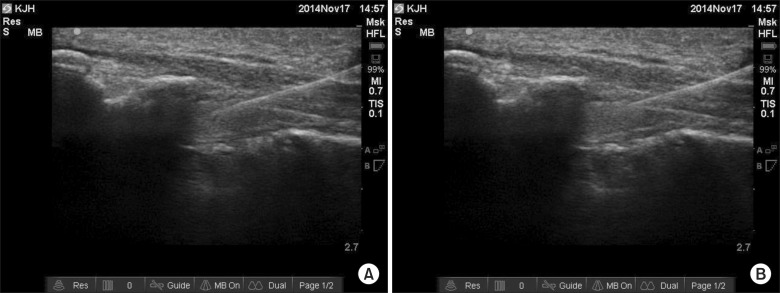
The patients were placed in the prone position with their heels rotated laterally and a cushion pillow was used as an iliac wedge to facilitate the exposure of the skin surface in the coccygeal area. After usual sterile preparation, the patients were scanned with a 13-6 MHz linear array transducer (EDGE® ultrasound machine, Sonosite inc., Bothell, Washington, USA) to identify the sacral hiatus and sacrococcygeal ligament. A transverse image for the sacral hiatus and dorsal sacrococcygeal ligament between the bilateral sacral cornua was obtained first, and transducer was then rotated by 90° to examine the longitudinal view of the sacral hiatus. Under real-time ultrasonography, a 25- gauge, 5 cm short-bevel needle (Disposable nerve blockade needle, UNISIS corp., Tokyo, Japan) was inserted and advanced at an approximate 45° angle until approaching the dorsal sacrococcygeal ligament, with a hyperechoic band like structure, using the in-plane technique. In Group 1, the needle was advanced 1 cm further into the sacral canal after piercing the sacrococcygeal ligament (Fig. 1A). In Group 2, the needle advancement was terminated right after penetrating the sacrococcygeal ligament (Fig. 1B). After confirming negative aspiration of cerebrospinal fluid and blood, the same solution containing 5.5 ml of 0.2% ropivacaine, 4 ml of contrast dye, and 0.5 ml of hyaluronidase 750 IU (a total volume of 10 ml) was injected in both groups. The position of the needle and dispersion of the contrast dye into the epidural space were observed through real- time fluoroscopic AP imaging, and the picture was also stored in a separate computer. When intravascular and intrathecal uptakes were detected under realtime fluoroscopy, the needle was immediately withdrawn and repositioned to ensure the safety of the intervention, and all cases were recorded. Additional complications associated with the procedure were also recorded. All the initial needle insertion procedures were performed according to the allocated groups, by one experienced pain physician, and another pain physician who was blinded to the study group subsequently injected the study solutions and observed the outcomes.
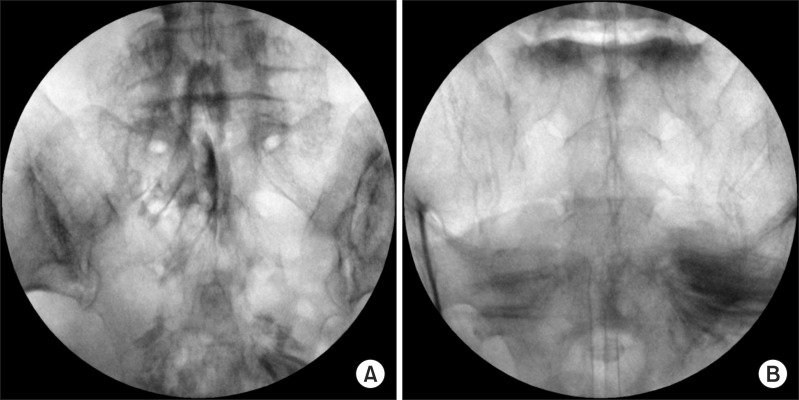
The real- time fluoroscopic images were analyzed by one pain physician who was blinded to the study group during the procedure, in order to discriminate proper dye spreading into the epidural space. A characteristic Christmas tree-like epidural and nerve root filling pattern without intravascular and intrathecal injections in the AP image was judged as a successful procedure (Fig. 2A). Conversely, an atypical contrast filling pattern instead of epidural spreading was considered to indicate a failed procedure (Fig. 2B).
Sample size was predetermined by Proportions Sample Size using SigmaPlot 12.5 (Systat Software Inc., San Jose, USA). Based on previously published data [2,3,4,5], we assumed that the success rate of the ultrasound-guided epidural injection through the conventional technique would be 70%, and determined that a difference of 25% would be of clinical significance. A total of 43 patients were required with a significance level of 0.05 (α = 0.05) and a power of 80% (β = 0.20). Taking into account the possibility of dropouts, a total of 50 patients were recruited for the study.
Statistical analysis was performed with SigmaPlot version 12.5. Continuous variables such as patient demographics and NRS were analyzed with Student's t-test or Mann-Whitney rank-sum test after normality test. And the success rates of the procedure and incidences of complications were analyzed using Fisher's exact test. All the data were expressed as the means ± standard deviations (SD), medians (25th-75th percentile), or the numbers of patients and percentages. A P value less than 0.05 was considered statistically significant.
Go to : 
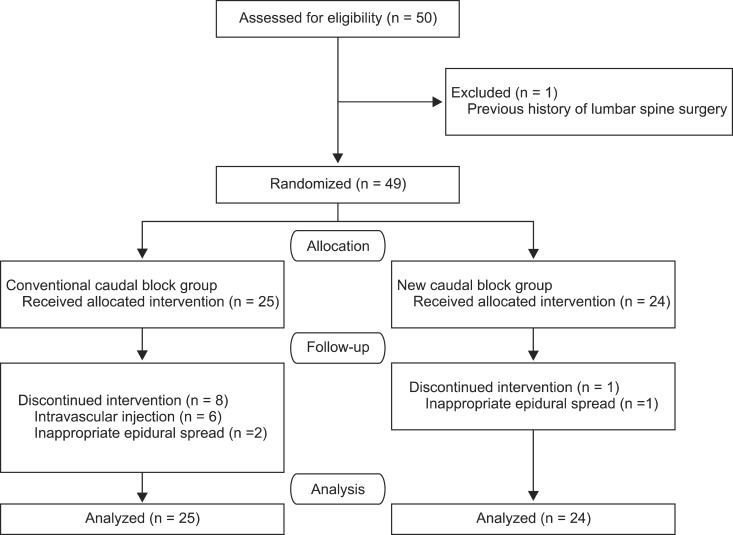
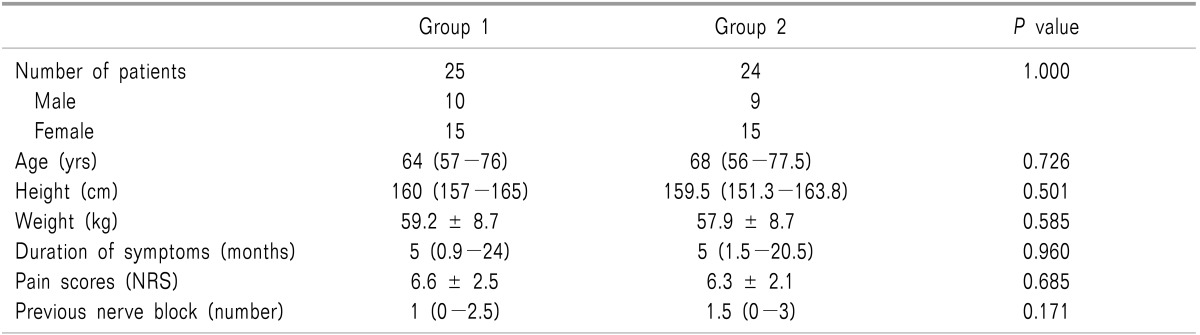
Fifty patients were recruited, and 49 completed the study. One patient with a previous surgical history of lumbar spine fusion was excluded from the study (Fig. 3). There were no significant differences between the two groups in terms of patient demographics, duration of the patients' symptoms, pain scores, or numbers of previous nerve block (Table 1). In the new method group (group 2), success rate of caudal injection was significantly higher than in the conventional method group (group 1) (P < 0.05; Table 2).
The number and causes of failed cases are summarized in Table 3. A total of 3 patients (two from Group 1 and one from Group 2) presented an atypical contrast filling pattern rather than an epidural spreading pattern, and were therefore considered failed cases. The incidences of intravascular injections confirmed by fluoroscopy were 24.0% (6 of 25) in Group 1, and there was no case of intravascular injection in Group 2 (P < 0.05; Table 3). Only 1 of the 6 patients presenting intravascular uptake of contrast dye had shown positive blood aspiration previously, and the procedure was successfully completed in all 6 patients on the second attempt. No intrathecal injection was found in either of the two groups.
Go to : 
Caudal steroid injection has been performed increasingly commonly, as it is easy to perform and relatively safe, presenting a lower risk of accidental dural puncture than other epidural techniques, such as interlaminar and transforaminal approaches [1]. Furthermore, caudal injections with or without steroids have been proven effective for pain relief and improvement of patients' functional status by a few prior studies [7,8,9,10,11]. Although caudal injection has generally been accepted as a safe procedure, major complications have been reported during and after caudal injections [12,13,14]. One of the critical complications following conventional caudal epidural injections is intravascular injection. According to other reported data, the incidence of accidental intravascular needle placement during a conventional caudal injection ranges widely, between 11-42% [2,3,4,5]. Consistently, in this study, intravascular contrast fillings were observed in 6 of 25 patients (24.0%) in the conventional method group (Group 1). On the contrary, no intravascular injection was detected in the new caudal injection group (Group 2), in which the needle only penetrated the sacrococcygeal ligament without being inserted into the sacral canal. The new method seems to present few risks of intravascular penetration caused by anatomical characteristics of the epidural venous plexus in the sacral canal. The epidural venous plexus is gathered in the anterior part of the sacral canal and generally ends at the S4 level or lower [15,16]. Considering the kyphotic feature of the sacrum and sacral canal, in the conventional caudal technique, no matter how the angle of the needle insertion is lowered to nearly 0°, the needle may be destined to be located in the anteriorly vessel-rich zone of the sacral canal. Therefore, the key to safe caudal injection is not to advance the needle into the sacral canal more than necessary. This concept was the starting-point for this study.
The sacral canal is an extension of the vertebral canal, which lodges 5 pairs of sacral nerves, and the dural sac is generally terminated at the 2nd sacral vertebra in adults. However, a cadaveric anatomical study of 49 Indian male adults reported terminations of the dura at the 3rd sacral vertebra level in about 8%, suggesting a potential risk of accidental dural puncture during caudal injections [17]. In another study analyzing lumbar magnetic resonance images, 22 (0.8%) of 2669 Korean patients had a dural sac and spinal canal that ended at or below S3 [18]. Meanwhile, inadvertent dural puncture can occur from misidentification of the sacral hiatus in patients with defects of the dorsal wall of the sacral canal. Aggarwal et al. [19] reported various types of sacral canal defects such as fusion failure of the sacral lamina and partial agenesis in 7.89% of 114 adult cadavers. Hence, pain physicians who perform the conventional caudal injection technique should be aware of the potential risk of dural puncture in some patients.
In our study, the needle insertion procedures were performed under ultrasound guidance, and the needles were successfully inserted through the sacral hiatus at the first attempt in all patients. In fact, the failure rate of the traditional caudal injection technique, preformed blindly, has been reported to reach up to 25% despite the various supporting methods available, such as the "Whoosh test" and nerve stimulation method [20,21,22]. The main causes of failure can include: 1) failure to identify the sacral hiatus due to uncertain surface anatomy or anatomical variation, 2) difficulty of inserting the needle through a too narrow sacral hiatus, and 3) impossiblity of advancing the needle into a sacral canal with a small AP diameter. Ultrasound use has great potential to improve the success rate of caudal injections, as it facilitates accurate needle placement by enabling identification of the location and morphology of the sacral hiatus, which is the hypoechoic space between the hyperechoic dorsal sacrococcygeal ligament and the bony wall of the sacrum, and making it possible to adjust the optimal angle of the needle insertion for unobstructed advancement. Ultrasound use has proved effective in guiding successful caudal epidural needle placement by a number of studies [23,24].
The authors of this study believed that fluoroscopic confirmation should be considered to improve the patients' outcomes and to reduce the risks of unexpected complications during conventional caudal injections, as ultrasound cannot evidence inadvertent intravascular injection and dural puncture, which are hidden by the bony sacral canal. In our institution, the routine method involves needle insertion through the sacral hiatus initiated under ultrasound, followed by final confirmation with contrast-dyed fluoroscopy. Our results demonstrate that the new caudal injection method is a reliable alternative that may not require fluoroscopic confirmation leading to unwelcome radiation exposure, as it presents low risks of intravascular injection or dural puncture. Moreover, this new caudal injection method requiring ultrasound only can be safely applied to patients as a bedside treatment tool.
In our study, a successful caudal block was defined as epidural and nerve root filling without intravascular and intrathecal injection during the procedure. Three of the patients, who were considered to present epidural spread failure, showed an atypical contrasted pattern rather than epidural filling (Fig. 2). The atypical patterns might have come from the various extents of the epidural pathologies including epidural adhesion and fibrosis. However, this could not be investigated clearly as not all patients had received radiologic evaluations before the procedure, although epidural pathologies cannot be fully evaluated with radiology. It is a limitation of this study that the placement of contrast at the pathologic nerve-root interface or inflamed lumbosacral root might also have to be evaluated for therapeutically-meaningful caudal epidural injections. In addition, the short and long-term therapeutic effects from the procedure were not investigated in this study.
In conclusion, the new method of caudal epidural injection, which consists in performing the injection right after penetrating the sacrococcygeal ligament, is a reliable alternative, with a higher success rate and lower risk of accidental intravascular injection than the conventional technique. Further-than-necessary advancement of the needle into the sacral canal should be avoided for safety reasons.
Go to : 
References
1. Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Fellows B. A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain Physician. 2012; 15:131–140. PMID: 22430650.
2. Sullivan WJ, Willick SE, Chira-Adisai W, Zuhosky J, Tyburski M, Dreyfuss P, et al. Incidence of intravascular uptake in lumbar spinal injection procedures. Spine (Phila Pa 1976). 2000; 25:481–486. PMID: 10707395.

3. Fukazawa K, Matsuki Y, Ueno H, Hosokawa T, Hirose M. Risk factors related to accidental intravascular injection during caudal anesthesia. J Anesth. 2014; 28:940–943. PMID: 24823700.

4. Manchikanti L, Cash KA, Pampati V, McManus CD, Damron KS. Evaluation of fluoroscopically guided caudal epidural injections. Pain Physician. 2004; 7:81–92. PMID: 16868617.
5. Ergin A, Yanarates O, Sizlan A, Orhan ME, Kurt E, Guzeldemir ME. Accuracy of caudal epidural injection: the importance of real-time imaging. Pain Pract. 2005; 5:251–254. PMID: 17147588.

6. Kim SG, Yang JY, Kim do W, Lee YJ. Inadvertent dural puncture during caudal approach by the introducer needle for epidural adhesiolysis caused by anatomical variation. Korean J Pain. 2013; 26:203–206. PMID: 23614088.

7. Parr AT, Manchikanti L, Hameed H, Conn A, Manchikanti KN, Benyamin RM, et al. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician. 2012; 15:E159–E198. PMID: 22622911.
8. Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Effect of fluoroscopically guided caudal epidural steroid or local anesthetic injections in the treatment of lumbar disc herniation and radiculitis: a randomized, controlled, double blind trial with a two-year follow-up. Pain Physician. 2012; 15:273–286. PMID: 22828681.
9. Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Results of 2-year follow-up of a randomized, doubleblind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician. 2012; 15:371–384. PMID: 22996849.
10. Manchikanti L, Cash KA, McManus CD, Pampati V. Fluoroscopic caudal epidural injections in managing chronic axial low back pain without disc herniation, radiculitis, or facet joint pain. J Pain Res. 2012; 5:381–390. PMID: 23091395.

11. Botwin K, Brown LA, Fishman M, Rao S. Fluoroscopically guided caudal epidural steroid injections in degenerative lumbar spine stenosis. Pain Physician. 2007; 10:547–558. PMID: 17660853.
12. Dere K, Akbas M, Bicerer E, Ozkan S, Dagli G. A complication during caudal steroid injection. J Back Musculoskelet Rehabil. 2009; 22:227–229. PMID: 20023355.

13. McGrath JM, Schaefer MP, Malkamaki DM. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011; 12:726–731. PMID: 21392252.

14. Yue WM, Tan SB. Distant skip level discitis and vertebral osteomyelitis after caudal epidural injection: a case report of a rare complication of epidural injections. Spine (Phila Pa 1976). 2003; 28:E209–E211. PMID: 12782996.

16. Yoon JS, Sim KH, Kim SJ, Kim WS, Koh SB, Kim BJ. The feasibility of color Doppler ultrasonography for caudal epidural steroid injection. Pain. 2005; 118:210–214. PMID: 16213088.

17. Aggarwal A, Kaur H, Batra YK, Aggarwal AK, Rajeev S, Sahni D. Anatomic consideration of caudal epidural space: a cadaver study. Clin Anat. 2009; 22:730–737. PMID: 19637298.

18. Joo J, Kim J, Lee J. The prevalence of anatomical variations that can cause inadvertent dural puncture when performing caudal block in Koreans: a study using magnetic resonance imaging. Anaesthesia. 2010; 65:23–26. PMID: 19922508.

19. Aggarwal A, Aggarwal A, Harjeet , Sahni D. Morphometry of sacral hiatus and its clinical relevance in caudal epidural block. Surg Radiol Anat. 2009; 31:793–800. PMID: 19578805.

20. Stitz MY, Sommer HM. Accuracy of blind versus fluoroscopically guided caudal epidural injection. Spine (Phila Pa 1976). 1999; 24:1371–1376. PMID: 10404581.

21. Lewis MP, Thomas P, Wilson LF, Mulholland RC. The 'whoosh' test. A clinical test to confirm correct needle placement in caudal epidural injections. Anaesthesia. 1992; 47:57–58. PMID: 1536408.
22. Tsui BC, Tarkkila P, Gupta S, Kearney R. Confirmation of caudal needle placement using nerve stimulation. Anesthesiology. 1999; 91:374–378. PMID: 10443599.

23. Nikooseresht M, Hashemi M, Mohajerani SA, Shahandeh F, Agah M. Ultrasound as a screening tool for performing caudal epidural injections. Iran J Radiol. 2014; 11:e13262. PMID: 25035698.

24. Chen CP, Tang SF, Hsu TC, Tsai WC, Liu HP, Chen MJ, et al. Ultrasound guidance in caudal epidural needle placement. Anesthesiology. 2004; 101:181–184. PMID: 15220789.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download