Abstract
Background
Epidural injections are performed utilizing 3 approaches in the lumbar spine: caudal, interlaminar, and transforaminal. The literature on the efficacy of epidural injections has been sporadic. There are few high-quality randomized trials performed under fluoroscopy in managing disc herniation that have a long-term follow-up and appropriate outcome parameters. There is also a lack of literature comparing the efficacy of these 3 approaches.
Methods
This manuscript analyzes data from 3 randomized controlled trials that assessed a total of 360 patients with lumbar disc herniation. There were 120 patients per trial either receiving local anesthetic alone (60 patients) or local anesthetic with steroids (60 patients).
Results
Analysis showed similar efficacy for caudal, interlaminar, and transforaminal approaches in managing chronic pain and disability from disc herniation. The analysis of caudal epidural injections showed the potential superiority of steroids compared with local anesthetic alone a 2-year follow-up, based on the average relief per procedure. In the interlaminar group, results were somewhat superior for pain relief in the steroid group at 6 months and functional status at 12 months. Interlaminar epidurals provided improvement in a significantly higher proportion of patients. The proportion of patients nonresponsive to initial injections was also lower in the group for local anesthetic with steroid in the interlaminar trial.
Conclusions
The results of this assessment show significant improvement in patients suffering from chronic lumbar disc herniation with 3 lumbar epidural approaches with local anesthetic alone, or using steroids with long-term follow-up of up to 2 years, in a contemporary interventional pain management setting.
The 2002, the Spine Patient Outcomes Research Trial (SPORT) was designed to prospectively collect primary data from patients identified as potential surgical candidates diagnosed with lumbar intervertebral disc herniation along with spinal stenosis and degenerative spondylolisthesis [1]. In a subgroup analysis of the SPORT trial, Radcliff et al. [2] suggested that epidural steroid injections are associated with a surgical avoidance rate of 41%. The results of SPORT also showed that disc herniation was seen in 38% of patients at L4-5 and 53% to 56% of patients at L5-S1 [3]. A majority of the discs in this assessment were extruded, ranging from 64% to 67%. Sequestered discs occurred in only 7% or 8% of the sample; whereas protruding discs were 26% to 29%. This is the latest contribution to the voluminous literature from the past 80 years or so devoted to the diagnosis and management of disc herniation as first described by Mixter and Barr in 1934 [4]. As shown in the SPORT trial, all patients with lumbar disc herniation or radiculopathy do not require surgical intervention, and multiple studies have shown that surgery may be avoided with epidural injections, admittedly at the variable rate of 41%-56% [2,5]. Consequently, multiple minimally invasive treatments, including epidural injections, are applied in addition to conservative management.
Epidural injections are one of the most commonly performed interventions for managing disc herniation [6,7,8,9,10,11,12,13]. Epidural injections are performed in the lumbar spine utilizing 3 different approaches: caudal, interlaminar, and transforaminal. These 3 approaches utilize different techniques with certain advantages and disadvantages, with potentially different outcomes based on the level of structural abnormalities [8,9,10,11,12,13]. The utilization of surgical and interventional techniques has been increasing rapidly with epidural injections showing an increase of 130% per 100,000 fee-for-service Medicare recipients [6,7]. From 2000 to 2011 lumbar interlaminar epidural injections increased by 25% [7]. In contrast, the increase in transforaminal epidural injections during this same period was almost 27 times higher at a rate of 665% when compared to both lumbar interlaminar and caudal epidural injections combined. Increases of utilization for other interventional techniques also have been higher, with a 331% increase for sacroiliac joint injections followed by a 308% increase for facet joint interventions, and a 544% increase for lumbar facet joint radiofrequency thermoneurolysis [7].
The interlaminar approach is considered capable of delivering the medication closest to the assumed site of pathology, but the transforaminal approach is considered the most target-specific modality requiring the smallest volume to reach the primary site of pathology. In contrast, caudal epidural injections require relatively large volumes and are associated with an alleged lack of specificity to the assumed site of pathology. Regardless, it is considered the safest and easiest approach, with minimal risk of inadvertent dural puncture, and is the preferred modality in postsurgery syndrome [8,9,10,11,12,13].
All 3 epidural injection approaches for lumbar disc herniation have been widely studied. Multiple systematic reviews and a number of randomized trials have been conducted assessing the effectiveness of all 3 epidural injection approaches [8,9,10,11,12,13]. The systematic reviews have provided highly variable results regarding the effectiveness of epidural injections for managing disc herniation [8,9,10,11,12,13]. Benyamin et al. [9] Parr et al. [10], and Manchikanti et al. [11,13] performed systematic reviews showing that multiple trials were performed with an inappropriate study design, a series of 3 epidural injections, and without fluoroscopy. Among the 3 epidural injection approaches, there were only 8 trials of moderate or high quality. All of these trials were performed under fluoroscopic visualization [8,13]. Furthermore, one randomized, controlled trial in each category was published by one group of authors, reporting the results of 120 patients in each trial with a 2-year follow-up [14,15,16]. Significant improvement in pain relief and functional status improvement of 50% or more was seen in 76%, 72%, and 77% of caudal, interlaminar, and transforaminal epidural injections respectively in responsive patients (at least 3 weeks of improvement with 2 initial procedures). The results showed the efficacy of epidural injections for all patients at 62%, 65%, and 61% of caudal, interlaminar, and transforaminal groups respectively, with significant improvement at 24 months. A cost utility analysis performed for caudal epidural injections for disc herniation showed a one-year quality-adjusted life year (QALY) of $2,206 [17].
The increasing prevalence of low back pain, coupled with the exponential increase of numerous modalities of management, increasing disability and soaring health care costs [6,7,8,18,19,20], epidural injections have faced significant criticism over the years despite emerging evidence [8,9,10,11,12,13,14,15,16,17]. Multiple trials of epidural injections by Manchikanti et al. [14,15,16,21,22,23,24,25,26,27,28] and a systematic review by Bicket et al. [29] have shown a lack of significant difference in outcomes between local anesthetics alone or local anesthetics with steroids.
Consequently, in this assessment we sought to evaluate the efficacy of 3 lumbar epidural injection approaches for managing chronic, intractable, persistent pain in the low back and lower extremities secondary to disc herniation or radiculitis after partial or nonresponsiveness of conservative management. The evaluation is based on 3 randomized trials with a 2-year follow-up and identical protocols [14,15,16].
The 3 trials [14,15,16] utilized for this assessment were conducted with the approval of the institutional review board (IRB) and were registered with the US Clinical Trial registry. Their assigned numbers were NCT00370799, NCT00681447 and NCT01052571. All trials were randomized, double-blind, active control trials utilizing local anesthetic alone or local anesthetic with steroids. These trials were performed in a private interventional pain management practice, a specialty referral center in the United States, by the same authors. The trials were conducted based on Consolidated Standards of Reporting Trials (CONSORT) guidance.
The trials and this analysis were conducted with internal resources.
The descriptions of participating patients, pre-enrollment evaluation, interventions, inclusion and exclusion criteria, description of interventions, additional interventions, co-interventions, objectives, outcomes, sample size calculation, randomization, sequence generation, allocation concealment, implementation, blinding and statistical methods were described in detail in the manuscripts [14,15,16].
All participating patients were recruited from those presenting at the practice for interventional pain management services.
The protocol consisted of 2 groups with 60 patients randomized into each group in each trial. The interventions were performed with local anesthetic alone or local anesthetic with steroid. For caudal epidural injections, a total of 10 ml of solution (10 ml of 0.5% lidocaine or 9 ml of lidocaine with 1 ml of steroid) was injected; for lumbar interlaminar epidural injections a total of 6 ml of solution (6 ml of 0.5% lidocaine or 5 ml of lidocaine with 1 ml of steroid) was injected; for lumbar transforaminal epidural injections a total volume of 2 ml was injected (1.5 ml of 1% preservative-free lidocaine along with 0.5 ml of sodium chloride solution or 3 mg of betamethasone).
Inclusion criteria included disc herniation or radiculitis in patients over 18 years of age with at least 6 months of function-limiting low back and lower extremity pain.
Exclusion criteria included previous lumbar surgery, radiculitis secondary to spinal stenosis, and radiculitis without disc herniation.
All procedures were performed in a sterile operating room under fluoroscopy by one physician (LM) with appropriate monitoring and intravenous sedation as indicated.
Caudal epidurals were performed by entering the epidural space through the sacral hiatus and confirmed by contrast medium injection. Lumbar interlaminar epidural injections were performed with the loss of resistance technique. All transforaminal epidural injections were performed by entering the foramen at one or 2 levels at the inferior aspect of the foramina at the lumbar levels and the sacral foramina with a 22-gauge Bella-D®-Coude® needle (Epimed International, Farmers Branch, TX) for lumbar levels; a 22-gauge spinal needle for sacral levels; and the caudal epidural space with an 18-gauge Tuohy needle through the sacral hiatus. Lumbar interlaminar injections used the loss of resistance technique with an 18-gauge Tuohy needle followed by confirmation by contrast medium injection.
The objective of this assessment was to evaluate and compare the efficacy of 3 lumbar epidural injection approaches using local anesthetic alone or local anesthetic with steroid for managing chronic low back and lower extremity pain secondary to disc herniation or radiculitis.
Patient outcomes were measured at baseline, 3, 6, 12, 18 and 24 months post-treatment. The outcomes measured were pain, using the Numeric Rating Scale (NRS) pain scale (0-10) [30]; functional assessment using the Oswestry Disability Index (ODI) (0-50 scale) [31]; employment status; opioid intake in terms of morphine equivalents. Thresholds for the minimum clinically important difference for ODI varied from a 4 to 15 point change from a total score of 50 and more recently, higher minimal improvements [14,15,16].
The sample size determination was based on previous assessments. Sample size calculation required a total of 110 patients with 55 patients in each group for each trial considering a 0.05, 2-sided significance level, with a power of 80%, and an allocation ratio of 1:1. Consequently, for the 3 trials, 120 patients were included in each trial.
Randomization was performed by computer-generated random allocations sequence by simple randomization.
The operating room nurse assisting with the procedure randomized the patients and prepared the drugs appropriately.
Group assignments were blinded to both the study patients and the medical personnel who administered the interventions. The injectates used for both groups were clear and indistinguishable from each other or covered to avoid identification.
For the present analysis, the Statistical Package for Social Sciences version 9.01 (SPSS Inc, Chicago, IL) was utilized. For categorical and continuous data comparison, Chi-square (Fisher test where necessary) and t test were used respectively. Because the outcome measures of the participants were measured at 6 points in time, repeated measures analysis of variance were performed with post hoc analysis with Bonferroni correction. A P value of less than 0.05 was considered significant.
An intent-to-treat analysis, which was performed after the sensitivity analysis in the original trials, was carried forward.
The trial recruitment period lasted from January 2007 through October 2009 for caudal epidural injections, January 2008 through May 2010 for interlaminar epidural injections, and January 2010 through December 2011 for transforaminal epidural injections.
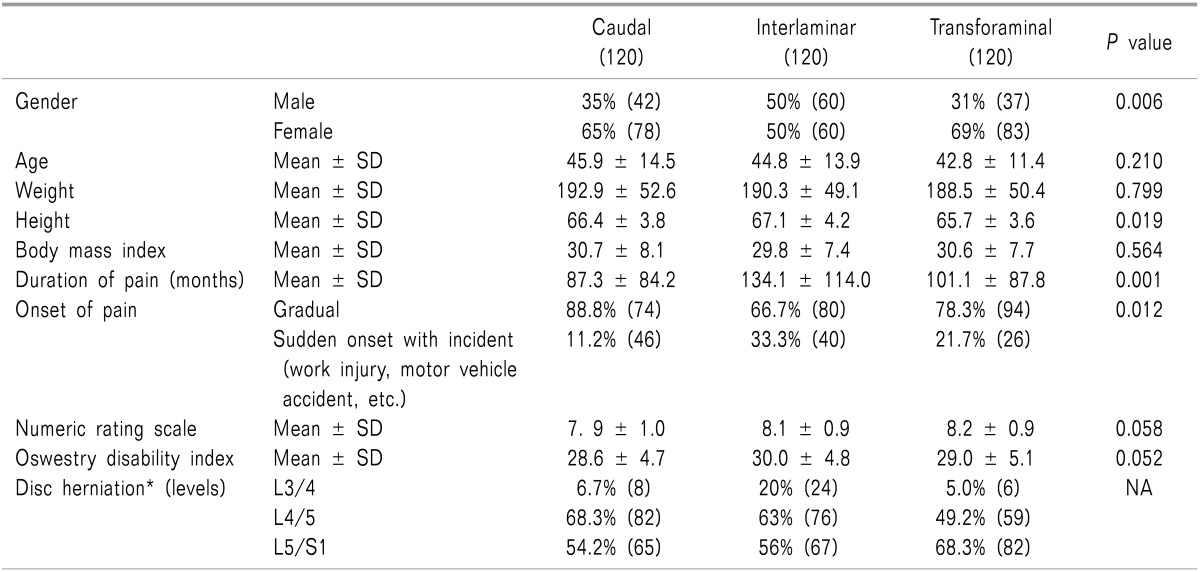
Table 1 shows the baseline demographic and clinical characteristics of each trial.
There were significant differences noted in the baseline characteristics among the 3 trials, for gender distribution, height, and duration of pain. Disc herniations in some patients were present at more than one level. Disc herniation at L5/S1 were 54% in the caudal trial, 56% in interlaminar trial and 68% in the transforaminal trial.
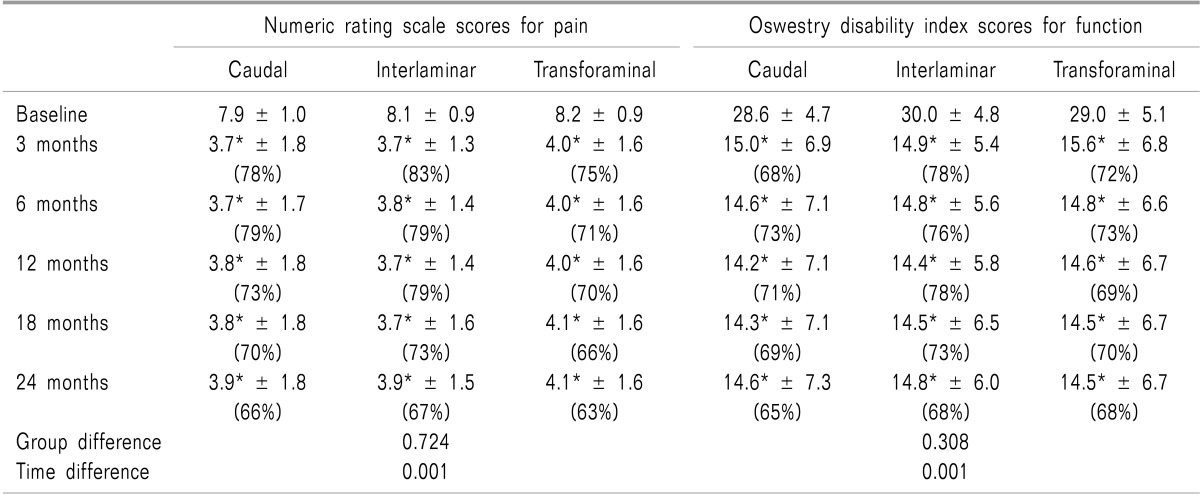
Pain relief and functional assessment: As shown in Table 2 and Fig. 1, 2, repeated measures ANOVA revealed time × factor (P < 0.001 for both NRS and ODI); however, among the 3 groups the effect was not found to be significant (P > 0.3 for VAS and ODI). Follow-up within a group pair-wise analysis revealed that NRS and ODI decreased significantly in all time intervals compared with baseline in the 3 groups (Table 2). A between-group analysis revealed that NRS and ODI scores were comparable in the 3 groups at all time intervals.
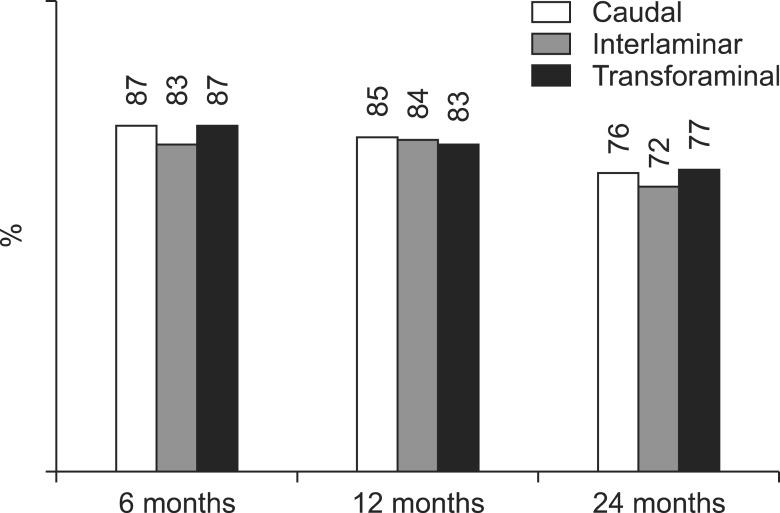
As shown in Fig. 3, 4, significant improvement was defined as 50% or more improvement in pain relief and functional status assessment. There was significant difference between interlaminar, and transforaminal at 12 months for steroid group (Fig. 3). There were no significant differences between groups (caudal, interlaminar, and transforaminal) at any of the time points as shown in Fig. 4. As shown in Fig. 5, significant pain relief at 12 months was 69%, 77% and 66%, and at 24 months was 63%, 65% and 61 for caudal, interlaminar and transforaminal epidurals respectively. Significant pain relief was comparable among the 3 groups at all follow-up points. Fig. 6 shows significant improvement for responsive patients only, with 76%, 72% and 77% at 24 months for caudal, interlaminar and transforaminal epidurals respectively; there was no significant difference among the 3 groups.
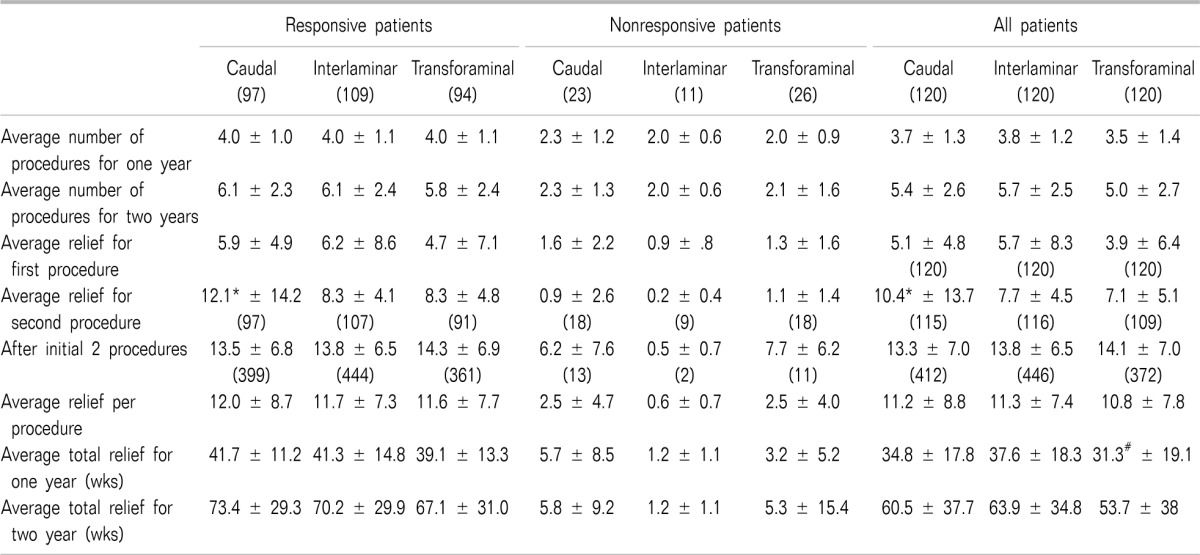
Therapeutic procedural characteristics for a period of 2 years for all 3 trials are shown in Table 3. The results showed 5 to 6 procedures over a period of 2 years with average relief for the first procedure of 4 to 6 weeks, with average relief for the second procedure of 7 to 12 weeks, and 13 to 14 weeks of average relief for the therapeutic phase after the first 2 procedures, per procedure. Average total relief for 2 years ranged from 67.1 ± 31 weeks in the transforaminal group, 70.2 ± 29.9 weeks in the interlaminar group, and 73.4 ± 29.3 weeks in the caudal group in responsive patients; whereas the average total relief of 2 years in all patients was 53.7 ± 38 weeks in the transforaminal group, 60.5 ± 37.7 weeks in the caudal group, and 63.9 ± 34.8 weeks in the interlaminar group.
In the management of chronic, persistent low back and lower extremity pain secondary to disc herniation and radiculitis, the present assessment comparing caudal, interlaminar, and transforaminal approaches to epidural injections in 3 large trials of 120 patients in each trial receiving either local anesthetic alone or local anesthetic with steroid with 60 patients in each group showed a lack of superiority for any of the approaches. A similar proportion of patients showed significant improvement in the 3 trials: 77% with caudal, 72% with interlaminar, and 80% with transforaminal approaches receiving local anesthetic alone, and 76% with caudal, 71% with interlaminar, and 73% with transforaminal with local anesthetic and steroid at 2 years. The number of procedures and the average relief for 2 years was also similar in all trials with local anesthetic alone or local anesthetic with steroid.
The analysis of caudal epidural injections of local anesthetic with steroid [14] showed the potential superiority for the addition of steroid compared with local anesthetic alone at 2-year follow-up based on the average relief per procedure. In addition, the lumbar interlaminar epidural injections trial [15] showed the potential superiority of local anesthetic with steroid for pain relief at 6 months and functional status at 12 months. Furthermore, the nonresponsive rate was significantly lower in the interlaminar group, 9% versus 19% in the caudal group and 22% in the transforaminal group. Consequently, it appears that patients in the interlaminar group who received steroid responded better, with only one nonresponsive patient compared to caudal epidural injections with steroids.
There was also no significant difference based on the levels of disc herniation. Based on the SPORT assessment of L4-5 disc herniations in 38%, one would expect superiority with lumbar interlaminar and transforaminal considering a targeted delivery rather than a large volume reaching the target site with caudal [3]. Disc herniations were noted at L4-5 in a significantly high proportion of patients in the caudal trial (68%) compared to interlaminar (80%) and transforaminal (50%) trials. One could postulate that L5-S1 disc herniation may respond well to a caudal epidural, but not L4-L5. This study shows otherwise, in that there were a larger proportion of patients with L4-L5 disc herniation in the caudal group than in the interlaminar group. Based on the lumbar interlaminar group, it appears that patients who were nonresponsive to epidural injections with local anesthetic alone may respond with the addition of a steroid. Thus, this assessment, based on the data from 3 large randomized trials, provides evidence that, in carefully selected patients, with repeat injections in contemporary interventional pain management settings under fluoroscopy, patients respond to both local anesthetic alone and local anesthetic with steroid in all 3 approaches in a similar fashion. Based on the frequency of epidural injections and the duration of relief, it appears that significant improvement lasts approximately 13 or 14 weeks. Consequently, it has been shown that over a period of 2 years, for multiple etiologies, approximately 6 epidural injections have been utilized in the group responsive to initial 2 procedures with at least 3 weeks of relief [14,15,16].
In the past, based on blind interlaminar trials, the evidence appeared to favor caudal epidural injections, whereas more recently it has been in favor of transforaminal epidural injections [8,10]. Now it appears, however, that based on large randomized controlled trials the evidence is the same for all 3 approaches. This assessment of trials performed with proper methodology in a practical setting provides appropriate information and facilitates the proper application of interventions to reduce a patient's pain, improve function, reduce drug use, and potentially return the patient to the work force. However, inappropriate provision of any type of intervention, specifically those that are not cost-effective, incurs substantial expenses [6,7,8,13,17,32,33,34]. Blinding would have been extremely difficult in one trial. Further, there was no placebo group in any of the trials. Systematic reviews undertaken without the proper utilization of criteria will ultimately be detrimental to both the patient and the economy of health care.
There has been only one randomized controlled trial, published by Ackerman and Ahmad [35], comparing caudal, interlaminar, and transforaminal approaches. This assessment showed the superiority for transforaminal over interlaminar and caudal, and interlaminar over caudal; however, the follow-up was for only 6 months and the trial was not of high quality [9,10,11,13].
In the era of evidence-based medicine and comparative effectiveness research, practical clinical trials with a pragmatic approach are considered to be clinically applicable and valid [7,8,9,10,11,17,32,33,34,36,37]. The trials utilized in this assessment met the essential criteria for practical clinical trials, with a measurement of effectiveness, rather than efficacy, which is considered to be more clinically applicable, resulting in practical implications and applications for interventional pain management providers [36,37].
This assessment may be criticized for multiple deficiencies, including the 3 separate randomized trials utilized in this analysis. The major deficiency of this assessment may be that these trials were conducted separately rather than as one trial; however, blinding would have been extremely difficult. There were no placebo groups in any of the trials. Placebo design and placebo use in interventional techniques continues to be widely debated. A placebo design for interventional techniques has been criticized for its inappropriate utilization in assessing epidural injections including caudal, interlaminar, and transforaminal approaches [7,8,9,10,11,38,39,40,41]. In addition, all the placebo-controlled trials in the interlaminar approach were in blind epidural groups with significant variability [42,43,44]. Two of these trials utilized an injection of sodium chloride solution into the interspinous ligament and compared that with epidural steroid injections [42,43]. One study was performed in 1973 [43] and other one was performed in 2005 [42]. These 2 studies reached different conclusions. One study commonly cited in systematic reviews and health policies that has obtained substantial publicity was published in the New England Journal of Medicine [44]. It utilized epidural saline versus steroid. In reference to caudal epidural, the only study with a placebo design was by Iversen et al. [40]. This study has been criticized for its inappropriate methodology and flawed conclusions. Recently, proper placebo design has been lauded for its use in transforaminal epidural injections and percutaneous adhesiolysis [38,41]. These properly conducted trials showed the appropriate effect of sodium chloride solution with injection into an inactive structure(s). Thus, the systematic reviews and opinions which equate local anesthetic with placebo are not only methodologically and conceptually inaccurate, but they also result in improper conclusions [12,32,33,34,40]. The role of placebo and appropriate interpretations of placebo have been extensively discussed [45,46,47]. Further, ample evidence has proved that inactive substances, when injected into active structures, invariably result in various types of clinical effects [8,9,10,11,12,13,14,15,16,21,22,23,24,25,26,27,28,47,48,49]. The injection of sodium chloride solution into an epidural space has been shown to be clinically effective in multiple studies [8,47]. Furthermore, local anesthetics also have shown long-term improvement or response equal to steroids in clinical and experimental settings [8,9,10,11,12,13,14,15,16,21,22,23,24,25,26,27,28]. Thus, it is imperative in interventional pain management to design a proper placebo study, with injection of inactive solutions into inactive structures. Further, it is also important to assess not only the differences among the 3 techniques, and the solutions injected, but also to extend the assessment at baseline to follow-up periods rather than depending on between the group or between the trial differences.
Epidural steroids in disc herniation and radiculitis are provided based on the pathophysiologic mechanism of inflammation [8,9,10,11,12,13,14,15,16,21,22,23,24,25,26,27,28]. Consequently, epidural steroids have been recommended as effective in disc herniation and radiculitis secondary to their antiinflammatory properties. However, emerging evidence also shows that local anesthetics with or without steroids are as equally effective as steroids in many settings [8,9,10,11,12,13,14,15,16,21,22,23,24,25,26,27,28].
The results of a 2 year follow-up of 3 randomized, double-blind, controlled trials, with a total of 360 patients with chronic persistent pain of disc herniation receiving either caudal, lumbar interlaminar or transforaminal epidural injections, showed similar efficacy of the 3 techniques with local anesthetic alone or local anesthetic with steroid. Caudal and interlaminar trials used in this assessment have shown some superiority of steroids over local anesthetic, at 3 and 6 month follow-up. Interlaminar with steroids were superior to transforaminal at 12-months.
ACKNOWLEDGEMENTS
The authors wish to thank Tom Prigge, MA, Alvaro F. Gómez, MA, Laurie Swick, BS for manuscript review, and Tonie M. Hatton and Diane E. Neihoff, transcriptionists, for their assistance in preparation of this manuscript.
References
1. Birkmeyer NJ, Weinstein JN, Tosteson AN, Tosteson TD, Skinner JS, Lurie JD, et al. Design of the Spine Patient outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2002; 27:1361–1372. PMID: 12065987.

2. Radcliff K, Hilibrand A, Lurie JD, Tosteson TD, Delasotta L, Rihn J, et al. The impact of epidural steroid injections on the outcomes of patients treated for lumbar disc herniation: a subgroup analysis of the SPORT trial. J Bone Joint Surg Am. 2012; 94:1353–1358. PMID: 22739998.

3. Lurie JD, Tosteson TD, Tosteson AN, Zhao W, Morgan TS, Abdu WA, et al. Surgical versus nonoperative treatment for lumbar disc herniation: eight-year results for the spine patient outcomes research trial. Spine (Phila Pa 1976). 2014; 39:3–16. PMID: 24153171.
4. Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934; 211:210–215.

5. Manson NA, McKeon MD, Abraham EP. Transforaminal epidural steroid injections prevent the need for surgery in patients with sciatica secondary to lumbar disc herniation: a retrospective case series. Can J Surg. 2013; 56:89–96. PMID: 23351495.

6. Manchikanti L, Falco FJ, Singh V, Pampati V, Parr AT, Benyamin RM, et al. Utilization of interventional techniques in managing chronic pain in the Medicare population: analysis of growth patterns from 2000 to 2011. Pain Physician. 2012; 15:E969–E982. PMID: 23159982.
7. Manchikanti L, Helm Ii S, Singh V, Hirsch JA. Accountable interventional pain management: a collaboration among practitioners, patients, payers, and government. Pain Physician. 2013; 16:E635–E670. PMID: 24284849.
8. Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013; 16:S49–283. PMID: 23615883.
9. Benyamin RM, Manchikanti L, Parr AT, Diwan S, Singh V, Falco FJ, et al. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012; 15:E363–E404. PMID: 22828691.
10. Parr AT, Manchikanti L, Hameed H, Conn A, Manchikanti KN, Benyamin RM, et al. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician. 2012; 15:E159–E198. PMID: 22622911.
11. Manchikanti L, Buenaventura RM, Manchikanti KN, Ruan X, Gupta S, Smith HS, et al. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician. 2012; 15:E199–E245. PMID: 22622912.
12. Pinto RZ, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, et al. Epidural corticosteroid injections in the management of sciatica: a systematic review and metaanalysis. Ann Intern Med. 2012; 157:865–877. PMID: 23362516.

13. Manchikanti L, Benyamin RM, Falco FJ, Kaye AD, Hirsch JA. Do epidural injections provide short- and long-term relief for lumbar disc herniation? A systematic review. Clin Orthop Relat Res. 2014; [in press].

14. Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Effect of fluoroscopically guided caudal epidural steroid or local anesthetic injections in the treatment of lumbar disc herniation and radiculitis: a randomized, controlled, double blind trial with a two-year follow-up. Pain Physician. 2012; 15:273–286. PMID: 22828681.
15. Manchikanti L, Singh V, Cash KA, Pampati V, Falco FJ. A randomized, double-blind, active-control trial of the effectiveness of lumbar interlaminar epidural injections in disc herniation. Pain Physician. 2014; 17:E61–E74. PMID: 24452658.
16. Manchikanti L, Cash KA, Pampati V, Falco FJ. Transforaminal epidural injections in chronic lumbar disc herniation: a randomized, double-blind, active-control trial. Pain Physician. 2014; 17:E489–E501. PMID: 25054399.
17. Manchikanti L, Falco FJ, Pampati V, Cash KA, Benyamin RM, Hirsch JA. Cost utility analysis of caudal epidural injections in the treatment of lumbar disc herniation, axial or discogenic low back pain, central spinal stenosis, and post lumbar surgery syndrome. Pain Physician. 2013; 16:E129–E143. PMID: 23703415.
18. Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008; 299:656–664. PMID: 18270354.

19. US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013; 310:591–608. PMID: 23842577.
20. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012; 37:67–76. PMID: 21311399.
21. Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician. 2012; 15:371–384. PMID: 22996849.
22. Manchikanti L, Cash KA, McManus CD, Pampati V. Fluoroscopic caudal epidural injections in managing chronic axial low back pain without disc herniation, radiculitis, or facet joint pain. J Pain Res. 2012; 5:381–390. PMID: 23091395.

23. Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Fluoroscopic caudal epidural injections in managing post lumbar surgery syndrome: two-year results of a randomized, double-blind, active-control trial. Int J Med Sci. 2012; 9:582–591. PMID: 23028241.

24. Manchikanti L, Cash KA, McManus CD, Pampati V, Benyamin RM. A randomized, double-blind, active-controlled trial of fluoroscopic lumbar interlaminar epidural injections in chronic axial or discogenic low back pain: results of 2-year follow-up. Pain Physician. 2013; 16:E491–E504. PMID: 24077199.
25. Manchikanti L, Cash KA, McManus CD, Damron KS, Pampati V, Falco FJ. Lumbar interlaminar epidural injections in central spinal stenosis: preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012; 15:51–63. PMID: 22270738.
26. Manchikanti L, Cash KA, Pampati V, Malla Y. Two-year follow-up results of fluoroscopic cervical epidural injections in chronic axial or discogenic neck pain: a randomized, double-blind, controlled trial. Int J Med Sci. 2014; 11:309–320. PMID: 24578607.

27. Manchikanti L, Malla Y, Cash KA, McManus CD, Pampati V. Fluoroscopic epidural injections in cervical spinal stenosis: preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012; 15:E59–E70. PMID: 22270749.
28. Manchikanti L, Malla Y, Cash KA, McManus CD, Pampati V. Fluoroscopic cervical interlaminar epidural injections in managing chronic pain of cervical postsurgery syndrome: preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012; 15:13–25. PMID: 22270734.
29. Bicket MC, Gupta A, Brown CH 4th, Cohen SP. Epidural injections for spinal pain: a systematic review and metaanalysis evaluating the "control" injections in randomized controlled trials. Anesthesiology. 2013; 119:907–931. PMID: 24195874.
30. National Institutes of Health, Warren Grant Magnuson Clinical Center (US). Pain intensity instruments July 2003. 0-10 Numeric rating scale [Internet]. Bethesda (MA): Warren Grant Magnuson Clinical Center;2003. Available at http://www.mvltca.net/Presentations/mvltca.pdf.
31. Fairbank JC, Pynsent PB. The oswestry disability index. Spine (Phila Pa 1976). 2000; 25:2940–2952. PMID: 11074683.

32. Manchikanti L, Falco FJ, Benyamin RM, Helm S 2nd, Singh V, Hirsch JA. Value-based interventional pain management: a review of medicare national and local coverage determination policies. Pain Physician. 2013; 16:E145–E180. PMID: 23703416.
33. Chou R, Atlas SJ, Loeser JD, Rosenquist RW, Stanos SP. Guideline warfare over interventional therapies for low back pain: can we raise the level of discourse? J Pain. 2011; 12:833–839. PMID: 21742563.

34. Manchikanti L, Benyamin RM, Falco FJ, Caraway DL, Datta S, Hirsch JA. Guidelines warfare over interventional techniques: is there a lack of discourse or straw man? Pain Physician. 2012; 15:E1–26. PMID: 22270745.
35. Ackerman WE 3rd, Ahmad M. The efficacy of lumbar epidural steroid injections in patients with lumbar disc herniations. Anesth Analg. 2007; 104:1217–1222. PMID: 17456677.

36. Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003; 290:1624–1632. PMID: 14506122.
37. ICH Expert Working Group (CH). International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Choice of control group and related issues in clinical trials E10 [Internet]. Geneva: ICH Expert Working Group;2000. 7. 20. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E10/Step4/E10_Guideline.pdf.
38. Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med. 2010; 11:1149–1168. PMID: 20704666.

39. Karppinen J, Malmivaara A, Kurunlahti M, Kyllönen E, Pienimäki T, Nieminen P, et al. Periradicular infiltration for sciatica: a randomized controlled trial. Spine (Phila Pa 1976). 2001; 26:1059–1067. PMID: 11337625.
40. Iversen T, Solberg TK, Romner B, Wilsgaard T, Twisk J, Anke A, et al. Effect of caudal epidural steroid or saline injection in chronic lumbar radiculopathy: multicentre, blinded, randomised controlled trial. BMJ. 2011; 343:d5278. PMID: 21914755.

41. Gerdesmeyer L, Wagenpfeil S, Birkenmaier C, Veihelmann A, Hauschild M, Wagner K, et al. Percutaneous epidural lysis of adhesions in chronic lumbar radicular pain: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2013; 16:185–196. PMID: 23703406.
42. Arden NK, Price C, Reading I, Stubbing J, Hazelgrove J, Dunne C, et al. A multicentre randomized controlled trial of epidural corticosteroid injections for sciatica: the WEST study. Rheumatology (Oxford). 2005; 44:1399–1406. PMID: 16030082.

43. Dilke TF, Burry HC, Grahame R. Extradural corticosteroid injection in management of lumbar nerve root compression. Br Med J. 1973; 2:635–637. PMID: 4577015.

44. Carette S, Leclaire R, Marcoux S, Morin F, Blaise GA, St-Pierre A, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997; 336:1634–1640. PMID: 9171065.

45. Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010; CD003974. PMID: 20091554.

46. Howick J, Bishop FL, Heneghan C, Wolstenholme J, Stevens S, Hobbs FD, et al. Placebo use in the United kingdom: results from a national survey of primary care practitioners. PLoS One. 2013; 8:e58247. PMID: 23526969.

47. Manchikanti L, Giordano J, Fellows B, Hirsch JA. Placebo and nocebo in interventional pain management: a friend or a foe-or simply foes? Pain Physician. 2011; 14:E157–E175. PMID: 21412379.
48. Indahl A, Kaigle AM, Reikeräs O, Holm SH. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine (Phila Pa 1976). 1997; 22:2834–2840. PMID: 9431619.

49. Indahl A, Kaigle A, Reikerås O, Holm S. Electromyographic response of the porcine multifidus musculature after nerve stimulation. Spine (Phila Pa 1976). 1995; 20:2652–2658. PMID: 8747243.

Fig. 1
Illustration of average Numeric Rating Scale scores for pain at different follow-up points by type of epidural. *P value at different time intervals as compared with baseline (within group, pair-wise comparisons with Bonferroni correction) #1 P = 0.058, #2 P = 0.227, #3 P = 0.269, #4 P = 0.355, #5 P = 0.202, #6 P = 0.515 respectively, for between-group comparisons at specified time intervals with Bonferroni correction.

Fig. 2
Illustration of average Oswestry Disability Index scores for function at different follow-up points by type of epidural. *P value at different time intervals as compared with baseline (within group, pair-wise comparisons with Bonferroni correction) #1 P = 0.052, #2 P = 0.652, #3 P = 0.951, #4 P = 0.896, #5 P = 0.963, #6 P = 0.925 respectively, for between-group comparisons at specified time intervals with Bonferroni correction.
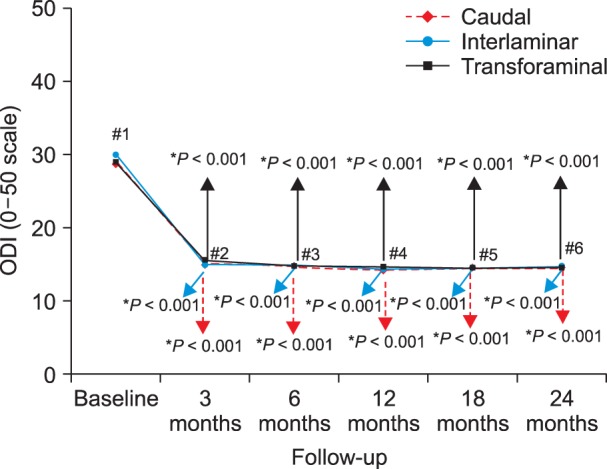
Fig. 3
Illustration of reduction (at least 50%) of Numeric Rating Scale scores for pain and Oswestry Disability Index scores for function from baseline (all patients).
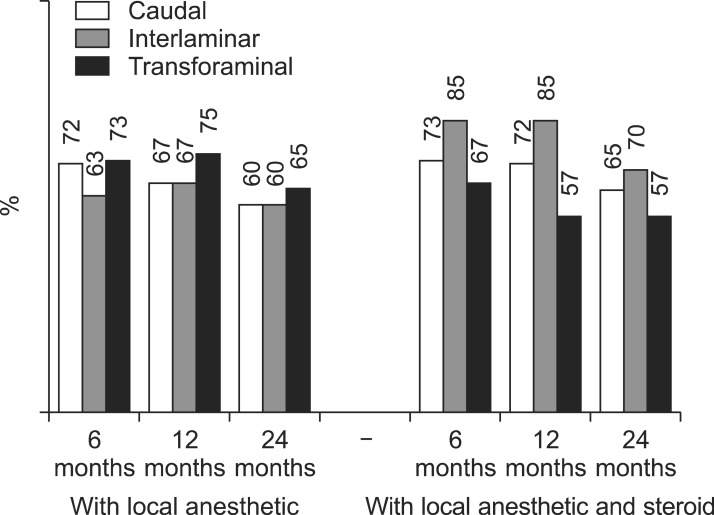
Fig. 4
Illustration of reduction (at least 50%) of Numeric Rating Scale scores for Pain and Oswestry Disability Index scores for function from baseline (only responsive patients).
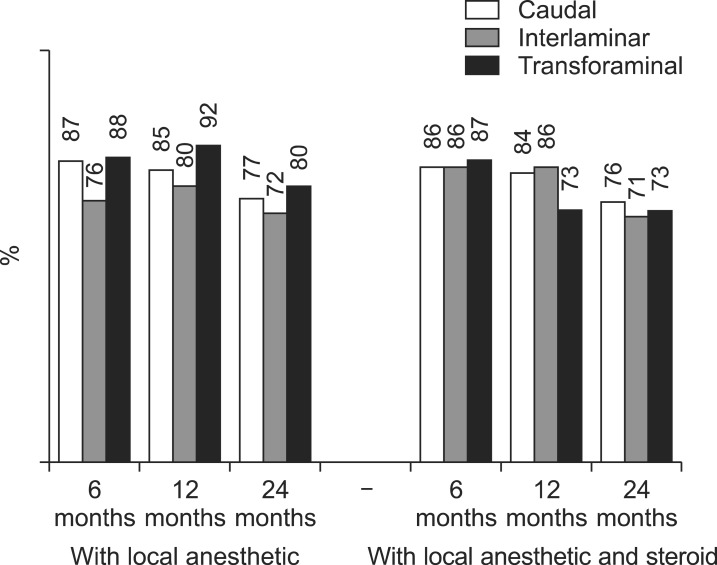
Fig. 5
Illustration of reduction (at least 50%) of Numeric Rating Scale scores for pain and Oswestry Disability Index scores for function from baseline (all patients).

Fig. 6
Illustration of reduction (at least 50%) of Numeric Rating Scale scores for pain and Oswestry Disability Index scores for function from baseline (only responsive patients).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download