Abstract
Background
Epidural steroid injection (ESI) is one of the most common procedures for patients presenting low back pain and radiculopathy. However, there is no clear consensus on what constitutes appropriate steroid use for ESIs. To investigate optimal steroid injection methods for ESIs, surveys were sent to all academic pain centers and selected private practices in Korea via e-mail.
Methods
Among 173 pain centers which requested the public health insurance reimbursements for their ESIs and were enrolled in the Korean Pain Society, 122 completed questionnaires were returned, for a rate of 70.5%; also returned were surveys from 39 academic programs and 85 private practices with response rates of 83.0% and 65.9%, respectively.
Results
More than half (55%) of Korean pain physicians used dexamethasone for ESIs. The minimum interval of subsequent ESIs at the academic institutions (3.1 weeks) and the private practices (2.1 weeks) were statistically different (P = 0.01).
Conclusions
Although there was a wide range of variation, there were no significant differences between the academic institutions and the private practices in terms of the types and single doses of steroids for ESIs, the annual dose of steroids, or the limitations of doses in the event of diabetes, with the exception of the minimum interval before the subsequent ESI.
Spinal pain is a leading cause of disability across the world. Lifetime prevalence rates of low back pain (LBP) range from 60 to 80% of all people in Korea [1,2]. For neck pain, the estimated range varies widely from 20 to 80% [3,4]. Among those who develop LBP, approximately 30% will develop chronic low back pain [5]. Compounding the high socioeconomic burden is the absence of any reliably effective treatment.
There are various schemes for categorizing chronic pain, with the most relevant likely being the classification of neuropathic or nociceptive pain, which influences clinical decisions for nearly all therapeutic options. Between 17 and 55% of patients with chronic LBP were found to have predominantly neuropathic characteristics [6-8]. Since Leivre et al. initiated epidural injections with the use of corticosteroids in 1953 [9], epidural steroid injections (ESIs) have been a cornerstone of a conservative management scheme of radiculopathy, at present being one of the most common procedures for patients presenting low back pain and radiculopathy around the world [10].
Epidural corticosteroid may provide significant pain relief by several mechanisms, i.e., inhibiting the production of arachidonic acid, which is the main mediator of inflammation [11,12]; inhibiting ectopic discharge from unmyelinated C fiber and injured nociceptive fiber; increasing the blood flow to ischemic nerve roots; and reliving central sensitization, such as through the "unwind" mechanism [13-15]. On the basis of these mechanisms of epidural steroid, previous reports suggested that ESIs are highly effective for short-term pain relief [16-19].
Complications associated with the epidural injection of corticosteroids are uncommon. However, a few studies have clarified that frequent ESIs suppress the adrenal gland and disturb the hormonal balance, resulting in an increased risk of developing Cushing's syndrome [20]. For diabetes patients, epidural steroid injections are assumed to increase blood sugar levels and the risk of glucose intolerance [21]. Neurologic injury due to the embolization of large steroid particles represents one of the most serious complications of ESIs [22,23]. Recently, the risk associated with the epidural administration of contaminated corticosteroids have been highlighted by the devastating outbreak of fungal meningitis in the United States [24].
Thus far, there is no clear consensus on what constitutes appropriate steroid use for ESIs [25], and little information with regard to recommendations or practice guidelines for the use of corticosteroids of ESIs is available. Differences in opinion as to what represents the optimal treatment extend to virtually all aspects of ESIs, including the type and dose of steroids, the frequency of administration, and decreasing doses of steroids used in patients with glucose intolerance, which forces pain physicians to perform ESIs with the arbitrary use of steroids. Therefore, considerable variations in the epidural administration of corticosteroids throughout Korea are being applied. To overcome this problem, the 'Special Group Publication Committee of ESIs' of the Korean Pain Society decided to conduct a survey on the corticosteroids used with ESIs in Korea. In attempts to investigate the most commonly used steroids and to determine whether there is any consensus as to what constitutes the optimal steroid injection for ESIs, a questionnaire was sent to all academic pain centers and selected private practices in Korea. Our goal in conducting this survey was to help establish a standard frame of reference for the performance of steroid use for ESIs in the treatment of chronic pain conditions in Korea.
The survey consisted of a total of 10 questions divided into three parts. The first part elicited information regarding the type and the demographics of the facility participating in the survey, including questions about the physician's experience and the number of blocks per day. The second section sought information about the methods withwhich epidural steroid injections were performed, the type of steroid injected, the dose of steroid per block, and the minimum interval between subsequent ESIs. Additionally, we asked about whether there were any differences in steroid dose according to their approaches, such as interlaminar and transforaminal approaches. The third section included questions about whether they decrease the dose of steroid in the patients with diabetes and if so, how much they decrease the dose of steroids injected into the epidural space for diabetic patients (Fig. 1).
From June 1 to July 31, 2013, surveys were sent via e-mail to the 173 pain centers which requested public health insurance reimbursements for their ESIs from the Health Insurance Review and Assessment Service in Korea and were enrolled in the Korean Pain Society; 47 anesthesia pain fellowship academic programs and 126 private pain practices were listed. In this study, 122 completed questionnaires were returned, for a rate of 71%; 39 academic programs and 85 private practices also responded, with response rates of 83% and 68%, respectively. Questions left unanswered or with ambiguous responses were not included in the data analysis.
The estimated standard deviation is abbreviated as S.D. When it is stated that there were no differences, this means that no statistically significant differences were found. Outcomes were summarized as a percentage of the institutions or the average from the number of institution ± the SD. In addition, we placed the answers into two groups: an academic center group and a private practice group, and compared them to each other.
A statistical analysis was performed using independent t-tests and descriptive analyses. Statistical analyses were performed using IBM® SPSS Statistics 20.
The academic institutes reported seeing an average of 40 ± 18 (range, 10-80) pain patients, whereas the corresponding number for the private practices was 39 ± 24 (range, 10-100).
Three private practices reported they did not use the interlaminar approach but only the transforaminal approach.
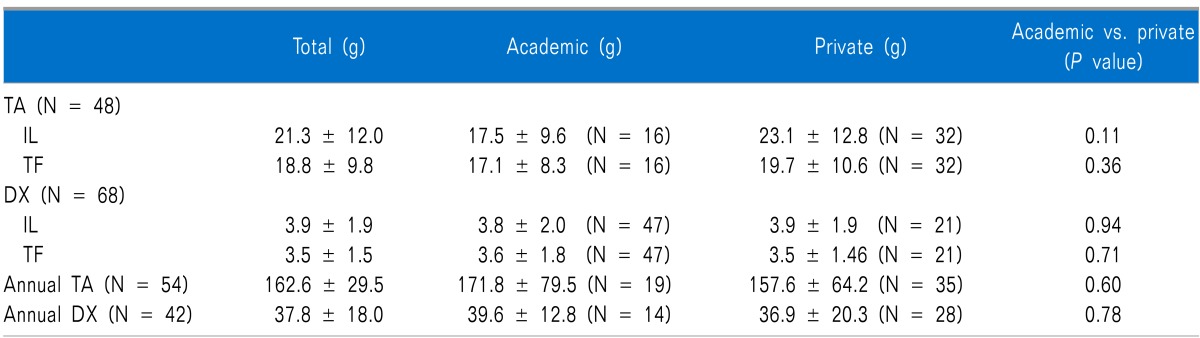
Among the 122 respondents, 39% (N = 48) reported that they injected triamcinolone (TA) for the interlaminar epidural approach and for the transforaminal approach (Table 1). For interlaminar epidural steroid injections, 21.3 ± 12.0 mg (range, 5.0-40.0) of TA was injected per injection. For the transforaminar epidural steroid injection, 18.8±9.8 mg (range, 5.0-40.0) was injected per injection. The maximal dose of TA for one block was less than 40 mg. There were no significant differences between the dose of TA injected into the epidural space via the interlaminar approach and the transforaminal approach and between the academic centers and the private practices.
More than 56% of the respondents (N = 68) injected dexamethasone for ESIs via the transforaminal and the interlaminar approaches. For the interlaminar approaches, dexamethasone injected into the epidural space was done so at a dose of 3.9 ± 1.9 mg (range, 1.0-8.0). For the transforaminal approaches, it was 3.5 ± 1.5 mg (range, 1.0-8.0). There were no significant differences between the doses of dexamethasone injected into the epidural space via the interlaminar approach and the transforaminal approach or between the academic centers and the private practice groups, either.
Four respondents, two academic institutions and two private practices, reported that they used both TA and dexamethasone for ESIs on a case by case basis.
Only 5% of the respondents (N = 6) reported that they injected betamethasone for ESIs, and its average dose was 1.3 ± 1.0 mg (range, 0.5-4.0).
Among all respondents, 29% (N =36) reported that they did not restrict the total dose of corticosteroids for ESI per year; 26% (N = 10) in the academic institutions, with 31% (N = 26) of the private practices also reporting this (Table 1). On the other hand, among the respondents who used TA for ESIs, 74% of academic institutions and 69% of the private practices reported that they put a limit on the annual doses of steroids. For TA, the respondents reported that that they a limited their annual doses of TA; 171.8 ± 79.5 (range, 25.0-300.0) mg/year in the academic institutions and 157.6 ± 64.2 (range, 25.0-300.0) mg/year in the private practices. For dexamethasone, the respondents reported that they limited their annual doses of dexamethasone; 39.6 ± 12.8 (range, 10.0-60.0) mg/year in the academic institutions and 36.9 ± 20.3 (range, 10.0 - 80.0) mg/year in the private practices. There were no statistical significant differences in the limits of annual doses of TA and dexamethasone between the academic institutions and the private practices (P = 0.60 and 0.78, respectively).
For ESIs performed subsequently, two-week intervals were the most popular (N = 49, 40%), followed by one-week intervals (N = 41, 34%). At the academic institutions, the minimum interval of ESIs for both the interlaminar or transforaminal approaches was 3.1 ± 3.5 (range, 1.0-20.0) weeks. At the private practices, the minimum interval of ESIs was 2.1 ± 1.5 (range, 1.0-10.0) weeks. There is a statistically significant difference in the minimum ESI intervals between the academic institutions and the private practices (P = 0.01).
Among all respondents, 59% (N = 72) reported that they used fewer corticosteroids for ESIs in patients with diabetes as compared to patients without diabetes. At both the academic institutions and private practices, physicians decreased the amounts of steroids by 0.6 (range, 0.49-0.70, 0.54-0.75, respectively) fold of the total dose used in the patients without diabetes. On the other hand, 34.1% (N = 41) of respondents reported that they did not reduce the steroid dose for ESIs in patients with diabetes. Finally, 7% (N = 9) of respondents replied that they never injected patients with diabetes with steroids for their ESIs.
In October 2012, fungal meningitis associated with contaminated glucocorticoid injections into the epidural space was reported in the New England Journal of Medicine [24]. An analysis of preliminary data from a large multistate database of fungal infections in the U.S. showed substantial morbidity and mortality. The infections were associated with injections of contaminated methylprednisolone acetate from a single compounding pharmacy. Then, another medication, TA acetonide from the same compounding pharmacy, was linked to a possible cause of fungal meningitis by the U.S. Food and Drug Administration (FDA). Although a patient with possible fungal meningitis was potentially associated with an epidural injection of TA acetonide, which is preservative-free and has not been imported to Korea, selecting the type of steroid is a very sensitive matter that is difficult to deal with for Korean pain physicians.
This study was carried out in a confused interim period as a research survey via e-mail to academic institutions and private practices. In total, 173 pain centers were listed (47 academic institutions and 126 private pain practices), and considering the response rate of 70%, this survey is assumed to represent the actual conditions pertaining to the use of steroids for epidural injections in Korea quite well. Specifically, the response rate of the private practices in our survey was about 68%, which was much higher than that in the similar surveys evaluating the technical aspects of ESI in the U.S. (36%) [26], reflecting their careful attention regarding the use of steroid for ESIs.
Although there was a wide range of variation, there were no significant differences between the academic institutions and the private practices in terms of the type and single doses of steroids for ESIs, the annual doses of steroids, and the limitations on doses for those with diabetes, with the exception of the minimum interval before the subsequent ESI. Nevertheless, the main finding in this survey was that there is no clear-cut consensus as to the selection of steroids and its optimum dose for ESIs.
The effects of epidural administered steroids stem from their ability to inhibit the synthesis of prostaglandins, their anti-inflammatory effects, and their ability to inhibit ectopic discharge from an injured sensory nerve [14,27]. However, data evaluating different types of steroid injections are mostly limited to underpowered, randomized or retrospective studies comparing particulate to nonparticulate steroids [28]. Among three randomized comparative-effectiveness studies comparing different steroid preparations, two studies reported there was no evidence that nonparticulate steroids such as dexamethasone at 10 mg were less effective than particulate steroids such as methylprednisolone, TA or betamethasone in lumbar transforaminar epidural injections [29,30]. However, Park et al. [31] compared 7.5 mg of dexamethasone to 40 mg into the epidural space in 103 patients with lumbar radiculopathy and reported that the nonparticulate steroid dexamethasone was statistically less effective than the particulate steroid TA in terms of pain relief. The most recent comparative study was a retrospective report that compared the efficacy of epidural dexamethasone at 10 mg to TA in patients who underwent lumbar transforaminar epidural injections, concluding that there was no evidence that nonparticulate steroids are less effective than particulate steroids [32].
With regard to the ideal dose of an epidural steroid, Wilkinson and Cohen [13] reported that particulate steroids such as TA or depomethylprednisolone are recommended at less than 40 mg for one interlaminar or transforaminar epidural block. They suggested that if more than 40 mg was injected at once, a ceiling effect could be anticipated. In accordance with their recommendation, our survey showed that the maximal dose of TA for a single epidural injection was never more than 40 mg in Korea. We found that 56% of pain physicians in all cases used the nonparticulate steroid dexamethasone for epidural steroid injections and that the dose of the injected dexamethasone was about 3.5 mg, showing no significant differences between interlaminar and transforaminar epidural injections or between an employed group and an unemployed group. A dose of dexamethasone of 3.5 mg is converted to 18.7 mg of triamcinolone [33], which is close to the dose of triamcinolone commonly used by the Korean pain physicians in our survey.
However, in a very recent systematic review and meta-analysis, nonsteroidal epidural injections were found to have benefits similar to those of steroidal injections, such as enhancing the blood flow to ischemic nerve roots, lysis of iatrogenic and inflammatory adhesions, and the washout of proinflammatory cytokines [29,34]. Bicket et al. [35] found that the benefits of favoring epidural nonsteroids over nonepidural injections is actually greater than the difference between an ESI and an epidural nonsteroid, suggesting that, at least in the short term, most of the benefit of an epidural injection derives from the solution itself, rather than the steroid. They also suggested that opportunities exist for clinicians and investigators to modify their approach to ESIs, such as reducing or even in some cases eliminating the steroid component of epidural injections in high-risk scenarios.
Therefore, thus far there has been conflicting evidence about what type of steroid and how much we should use for ESIs, and there is no consensus on the proper sort of steroids and/or amount(s) as yet.
The frequency and total number of injections have been considered as very important; however, they are the most controversial and poorly addressed issues [36]. In many cases, conclusions are based on flawed assumptions from non-existing evidence. Over the years, some authors have suggested three injections in a series irrespective of the patient's progress [37,38], whereas there are also proponents who propose that an unlimited number of injections with no established goals or parameters should be available. A limitation of 3 mg per kilogram of body weight of steroid or 210 mg per year in an average person and a lifetime dose of 420 mg of steroid also has been advocated [37]; however, this was done no scientific basis. The administration must be based solely on patients' responses, the safety profile of the drug, the experience of the patient, and pharmacological and chemical properties such as the duration of action and the suppression of adrenals. In our survey, 29% of respondents reported that they did not restrict the total dose of steroids for ESIs. In contrast, among all respondents, the majority reported they limited their annual dose TA to 162.6 mg/year, which was much lower than the dose of 210 mg previously suggested by Manchikanti [37].
Methylprednisolone appears to be sustained in the epidural space for more than two weeks, also inducing adrenocorticotropic hormonal depression for two weeks after an ESI [39]. For this reason, the minimum interval between ESIs would be considered as two weeks among the majority of pain physicians. In this survey, the minimum intervals before the subsequent ESI at the academic institutions and the private practices were more than two weeks, also showing a statistically significant difference (P = 0.01), at 3.1 and 2.1 weeks, respectably. The reason for the difference in the minimum interval before the subsequent ESI is suspected to be related to the better accessibility of the private practices relative to that of the academic institutions.
It is well known that the administration of a glucocorticoid reduces the hypoglycemic effect of insulin and interferes with blood glucose control in diabetic patients [21]. Following an injection of a depo-steroid, diabetic patients generally experience significant increases in their blood glucose levels and insulin requirements for one to two days after injection [25]. A study of 30 diabetic patients demonstrated significant changes in blood glucose levels (from 160 to 286) to that normalized within two days after an ESI [25]. There was no correlation between HbA1C levels before an injection and the response rates. Therefore, glucose levels in diabetic patients should be monitored closely during the first two days following any type of steroid injection. Patients need to be informed that an adjustment of their insulin dose may be required.
There remains no clear consensus to define epidural steroid doses thus far. However, according to the survey, more than half of Korean pain physicians reported that they use fewer steroids (an average of 0.6 fold of the total steroid dose used in patients without diabetes) for ESIs in patients with diabetes, without a statistical difference between the academic institutions and the private practices.
The results of our survey indicate that there is still no clear-cut consensus at either academic institutes or private practices with regard to the type, dosage, frequency, or total number of injections for ESIs. More than half (56%) of Korean pain physicians who responded to our survey selected dexamethasone for their ESIs. The minimum interval of subsequent ESIs at the academic institutions (3.1 weeks) and at the private practices (2.1 weeks) were more than two weeks in each case, but the difference was statistically significant (P = 0.01).
We hope that the information provided by our survey will be helpful for those who develop standards of reference that can be used when Korean pain physicians give epidural steroid injections in the future. Clearly, more research is needed to find the most effective and least harmful standard reference when utilizing ESIs.
References
1. Cho NH, Jung YO, Lim SH, Chung CK, Kim HA. The prevalence and risk factors of low back pain in rural community residents of Korea. Spine (Phila Pa 1976). 2012; 37:2001–2010. PMID: 22588379.

2. Baek SR, Lim JY, Lim JY, Park JH, Lee JJ, Lee SB, et al. Prevalence of musculoskeletal pain in an elderly Korean population: results from the Korean Longitudinal Study on Health and Aging (KLoSHA). Arch Gerontol Geriatr. 2010; 51:e46–e51. PMID: 20005585.

3. Son KM, Cho NH, Lim SH, Kim HA. Prevalence and risk factor of neck pain in elderly Korean community residents. J Korean Med Sci. 2013; 28:680–686. PMID: 23678258.

4. Koh MJ, Park SY, Woo YS, Kang SH, Park SH, Chun HJ, et al. Assessing the prevalence of recurrent neck and shoulder pain in Korean high school male students: a cross-sectional observational study. Korean J Pain. 2012; 25:161–167. PMID: 22787546.

5. Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ. 2008; 337:a171. PMID: 18614473.

6. El Sissi W, Arnaout A, Chaarani MW, Fouad M, El Assuity W, Zalzala M, et al. Prevalence of neuropathic pain among patients with chronic low-back pain in the Arabian Gulf Region assessed using the leeds assessment of neuropathic symptoms and signs pain scale. J Int Med Res. 2010; 38:2135–2145. PMID: 21227019.

7. Kaki AM, El-Yaski AZ, Youseif E. Identifying neuropathic pain among patients with chronic low-back pain: use of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale. Reg Anesth Pain Med. 2005; 30:422–428. PMID: 16135345.

8. Beith ID, Kemp A, Kenyon J, Prout M, Chestnut TJ. Identifying neuropathic back and leg pain: a cross-sectional study. Pain. 2011; 152:1511–1516. PMID: 21396774.

9. Lievre JA, Bloch-Michel H, Pean G, Uro J. L'hydrocortisone en injection locale. Rev Rhum Mal Osteoartic. 1953; 20:310–311.
10. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Growth of spinal interventional pain management techniques: analysis of utilization trends and Medicare expenditures 2000 to 2008. Spine (Phila Pa 1976). 2013; 38:157–168. PMID: 22781007.
11. Benzon HT. Epidural steroid injections for low back pain and lumbosacral radiculopathy. Pain. 1986; 24:277–295. PMID: 3008063.

12. Wilkinson IM, Cohen SP. Epidural steroid injections. Curr Pain Headache Rep. 2012; 16:50–59. PMID: 22090263.

13. Wilkinson I, Cohen SP. Epidural steroids for spinal pain and radiculopathy: a narrative, evidence-based review. Curr Opin Anaesthesiol. 2013; [in press].
14. Devor M, Govrin-Lippmann R, Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985; 22:127–137. PMID: 4047699.

15. Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990; 34:335–338. PMID: 2167604.

16. Diwan S, Manchikanti L, Benyamin RM, Bryce DA, Geffert S, Hameed H, et al. Effectiveness of cervical epidural injections in the management of chronic neck and upper extremity pain. Pain Physician. 2012; 15:E405–E434. PMID: 22828692.
17. Stafford MA, Peng P, Hill DA. Sciatica: a review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007; 99:461–473. PMID: 17704089.

18. Parr AT, Manchikanti L, Hameed H, Conn A, Manchikanti KN, Benyamin RM, et al. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician. 2012; 15:E159–E198. PMID: 22622911.
19. Quraishi NA. Transforaminal injection of corticosteroids for lumbar radiculopathy: systematic review and meta-analysis. Eur Spine J. 2012; 21:214–219. PMID: 21892774.

20. Jacobs S, Pullan PT, Potter JM, Shenfield GM. Adrenal suppression following extradural steroids. Anaesthesia. 1983; 38:953–956. PMID: 6314836.

21. Munck A. Glucocorticoid inhibition of glucose uptake by peripheral tissues: old and new evidence, molecular mechanisms, and physiological significance. Perspect Biol Med. 1971; 14:265–269. PMID: 5546253.

22. Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004; 4:468–474. PMID: 15246308.

23. Glaser SE, Falco F. Paraplegia following a thoracolumbar transforaminal epidural steroid injection. Pain Physician. 2005; 8:309–314. PMID: 16850088.
24. Lestner JM, Smith PB, Cohen-Wolkowiez M, Benjamin DK Jr, Hope WW. Antifungal agents and therapy for infants and children with invasive fungal infections: a pharmacological perspective. Br J Clin Pharmacol. 2013; 75:1381–1395. PMID: 23126319.

25. Even JL, Crosby CG, Song Y, McGirt MJ, Devin CJ. Effects of epidural steroid injections on blood glucose levels in patients with diabetes mellitus. Spine (Phila Pa 1976). 2012; 37:E46–E50. PMID: 21540770.

26. Cluff R, Mehio AK, Cohen SP, Chang Y, Sang CN, Stojanovic MP. The technical aspects of epidural steroid injections: a national survey. Anesth Analg. 2002; 95:403–408. PMID: 12145061.

27. Hirata F, Schiffmann E, Venkatasubramanian K, Salomon D, Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980; 77:2533–2536. PMID: 6930649.

28. Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: a comprehensive, evidence-based review. Reg Anesth Pain Med. 2013; 38:175–200. PMID: 23598728.
29. Kim D, Brown J. Efficacy and safety of lumbar epidural dexamethasone versus methylprednisolone in the treatment of lumbar radiculopathy: a comparison of soluble versus particulate steroids. Clin J Pain. 2011; 27:518–522. PMID: 21562412.

30. Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006; 7:237–242. PMID: 16712623.

31. Park CH, Lee SH, Kim BI. Comparison of the effectiveness of lumbar transforaminal epidural injection with particulate and nonparticulate corticosteroids in lumbar radiating pain. Pain Med. 2010; 11:1654–1658. PMID: 20807343.

32. El-Yahchouchi C, Geske JR, Carter RE, Diehn FE, Wald JT, Murthy NS, et al. The noninferiority of the nonparticulate steroid dexamethasone vs the particulate steroids betamethasone and triamcinolone in lumbar transforaminal epidural steroid injections. Pain Med. 2013; 14:1650–1657. PMID: 23899304.

33. Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977; 63:200–207. PMID: 888843.
34. Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine (Phila Pa 1976). 1998; 23:2538–2544. PMID: 9854752.
35. Bicket MC, Gupta A, Brown CH 4th, Cohen SP. Epidural injections for spinal pain: a systematic review and meta-analysis evaluating the "control" injections in randomized controlled trials. Anesthesiology. 2013; 119:907–931. PMID: 24195874.
36. Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009; 12:699–802. PMID: 19644537.
37. Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002; 5:182–199. PMID: 16902669.

38. McLain RF, Kapural L, Mekhail NA. Epidural steroid therapy for back and leg pain: mechanisms of action and efficacy. Spine J. 2005; 5:191–201. PMID: 15749619.

39. Bellini M, Barbieri M. Systemic effects of epidural steroid injections. Anaesthesiol Intensive Ther. 2013; 45:93–98. PMID: 23877903.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download