Abstract
Background
Lumbar transforaminal epidural steroid injections (TFESIs) are performed to provide symptom relief in patients with radicular pain. Recent articles suggested that injected volume itself have analgesic effects and higher volumes are associated with better outcomes. To date, few studies have been conducted to investigate the effects of volume. Therefore, well-designed controlled studies were necessary to confirm the effect of volume itself on pain relief. The purpose of this study was to examine the effectiveness of a forceful saline injection on lumbar TFESI using non-particulate steroids.
Methods
Fifty consecutive patients with lumbar radicular pain were enrolled. The participants were allocated into one of two groups: dexamethasone with volume (Group DV) and dexamethasone alone (Group DO). The volume was delivered by a forceful injection of 5ml of normal saline. The primary end-point for this study was a VAS pain score and modified MacNab score indicating the rate of effectiveness at the four-week follow-up.
Go to : 
Lumbar transforaminal epidural steroid injections (TFESIs) are performed to provide symptom relief in patients with radicular pain [1,2]. Radicular pain manifests when adhesions and inflammation in the epidural space stimulate nerve roots. Steroid-containing injectates are thought to reduce pain due to their anti-inflammatory properties and membrane-stabilizing effects. They has also been proved to be effective as a non-surgical option [3,4].
Recently two review articles suggested that the injected volume itself is associated with better outcomes. Rabinovitch et al. [5] demonstrated statistically significant associations between fluid amounts injected epidurally and pain relief in patients with radicular leg pain or lower back pain. These findings suggested that a larger amount of volume injected led to greater pain relief. Cohen et al. [1] also concluded that higher volumes are associated with better outcomes, and there is some evidence of epidural injections of nonsteroidal solutions having analgesic effects [6]. A proposed mechanism pertaining to this finding involves the injected fluid leading to the lysis of neural adhesions by means of stretching along the dura and nerve roots [7].
However, as the authors already admitted, these results should be interpreted cautiously. Many of these studies used heterogeneous methods, including various means of medication preparation and diffent procedural approaches. How injected volume was delivered was not specifically described, as none of the studies were designed to investigate the effects of the injected volume. Moreover, most of the procedures used were not under fluoroscopic guidance, posing a problem with accuracy.
Therefore, well-designed controlled studies are necessary to confirm the effect of the volume itself on pain relief. The purpose of this study was: to examine the effectiveness of a forceful saline injection on lumbar TFESI using non-particulate steroids.
Go to : 
Research participants included individuals complaining of moderate to severe lumbar radicular pain (VAS ≥ 4), with a diagnosis of herniated nucleus pulposus or spinal stenosis after a series of physical, neurologic and radiologic exams (MRI and CT) in an outpatient setting. Subjects with any history of spinal intervention or other similar procedures over the previous four weeks or those with oral steroid use, pregnancy, cognitive disorders, neuropsychiatric disease or anti-coagulant use were excluded. Our Institutional Review Board approved this research protocol, and written informed consent was obtained from every participant (ECT 13-14A-39).
Using outpatient visit dates, participants were allocated into one of two groups, termed the 1) dexamethasone with volume group (Group DV) and the 2) dexamethasone alone group (Group DO). Patients were placed in a prone position. Betadine was used to create a sterile field, and local anesthetic agents were injected at the needle insertion site. A 22-G Quincke spinal needle (spinal needle, TaeChang, Korea) was inserted using a preganglionic transforaminal approach with antero-posterior and lateral fluoroscopic assistance. About 1 ml of Contrast media (Pamiray, Dongkook, Korea) was injected to confirm expansion through the epidural space and the involvement of corresponding nerve roots. In the DV group, 5 ml of normal saline was forcefully injected first. After the disappearance of paresthesia or radiating pain, 3 ml of 0.33% lidocaine with 4 mg dexamethasone disodium phosphate (Dexamethasone, Yuhan, Korea) was injected. In the DO group, 3 ml of 0.33% lidocaine with 4 mg dexamethasone disodium phosphate was injected. The same procedure was utilized on the contralateral side for patients with bilateral symptoms. A syringe with a filter needle (Filter needle, Donghwa C&M, Korea) was used to avoid the risk of glass particles in cases of drugs contained in a glass ampoule.
Participants were seen four weeks post-procedure, and interviewed by phone when not available to meet in the clinic. Patients filled out a Visual Analog Scale (VAS) form both before and after the procedure. For a functional effectiveness analysis, a modified MacNab score was used with the following scale: 1) Excellent - Disappearance of pain and numbness, without any motor dysfunction. 2) Good - Disappearance of most primary symptoms, occasional pain, and ability to return to modified work. 3) Fair - Symptoms and functional capacity improvement, but still unable to participate in daily work. 4) Poor - No improvement or aggravation of symptoms, inability to participate in daily work. Effective treatment was defined as a score of "excellent" or "good."
The primary end-point for this study was a VAS pain score and modified MacNab score indicating the rate of effectiveness at the four-week follow-up. All statistical analyses were performed using statistical software (SPSS 18, Chicago, IL, USA). Data were presented as means ± standard deviations unless otherwise noted. Mann-Whitney U Tests were performed to compare continuous variables. Discrete variables were analyzed by means of chi-square tests. Results were considered statistically significant at a P value < 0.05.
Go to : 
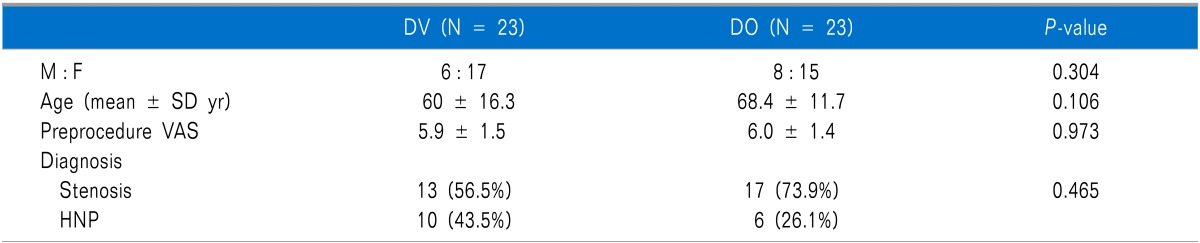
Fifty consecutive patients who attended outpatient office visits were divided into two groups of 25 patients each. In the DV group, two patients were lost to follow-up. In the DO group, one patient was lost to follow-up, and another patient withdrew during the follow-up period. Consequently, 23 patients remained in each group. There were no significant differences in terms of the mean age, gender, underlying disease or baseline VAS score between the two groups (Table 1).
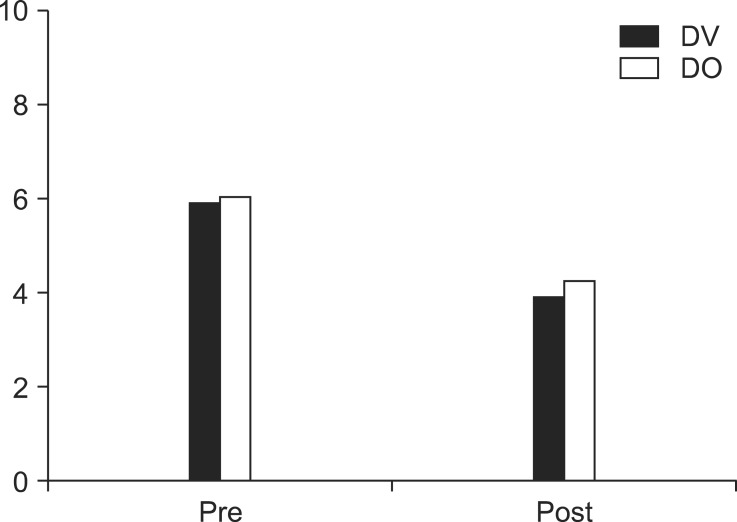
After four weeks of treatment, all groups demonstrated a decrease in VAS, but there were no significant post-procedural VAS differences between the two groups (P = .252) (Fig. 1).
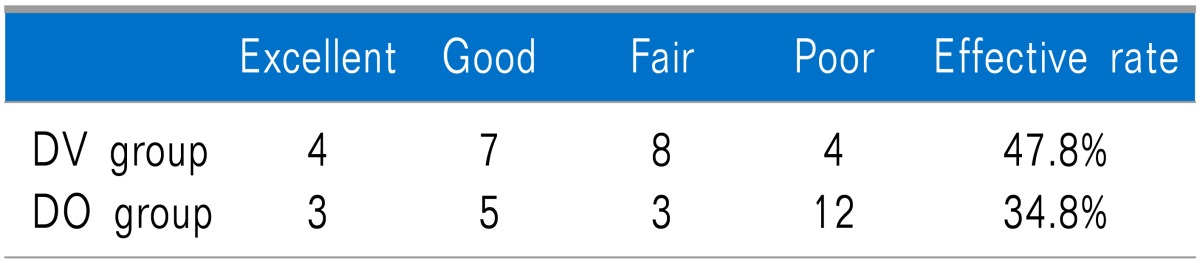
According to the modified MacNab score, efficacy rates were 47.8% in the DV group, and 34.8% in the DO group. These differences were not statistically significant (P = .117) (Table 2).
No adverse events were reported in this study.
Go to : 
According to this four-week treatment study, both groups reported improved pain reduction and functional effectiveness. However, the differences between the DV and DO groups were not significant in terms of outcome measures.
A few controlled studies have attempted to confirm the effect of the volume itself on pain relief. One randomized controlled study investigated the efficacy of percutaneous epidural adhesiolysis by a forceful injection of a high volume of saline via a caudal approach [8]. It concluded that VAS scores and oral opioid intake were significantly decreased in the study group at one and six months. Another retrospective study reported that no differences were found between small (2 ml) and large (8 ml) volume transforaminal epidural blocks [9]. The authors focused on the dilution effect on inflammatory mediators and not on epidural adhesiolysis. The effect of the volume may be masked by use of triamcinolone, which has excellent efficacy itself.
Our study was designed to inject identical solutions containing the same dose and concentration of local anesthetics, as well as equivalent doses of steroids via a transforaminal approach under fluoroscopic guidance. The only difference between the DV and DO groups was an additional injection of normal saline. We chose a combination of lidocaine and dexamethasone because it is considered to be the safest combination in terms of a precipitate risk of embolization [10,11].
Two remaining issues were the volume of injectate and how the saline was to be injected. No consensus exists regarding the optimal volume for a transforaminal approach. Furman et al. investigated the amount of volume needed to reach specific anatomic landmarks under fluoroscopic guidance [12]. Withr 4 ml of injected contrast, 93% of L-TFESI reached both the superior aspect of the superior intervertebral disk and the inferior aspect of the inferior intervertebral disk. Based on Furman's findings, we considered that at least 4 ml of volume would be needed to surround the affected nerve roots. It was also considered that with greater volumes injected via the transforaminal approach, more paresthesia or radiating pain could be experienced by patients. The minimum volume covering the affected area was thought to be 4 ml. Thus, an injection of 5 ml of normal saline was used in our study.
The proposed mechanism of action of the injected volume was such that the injected fluid stretch the dura and nerve roots back and forth, leading to the lysis of neural adhesions [7], which is very similar to that of epidural adhesiolysis. There has been recent speculation surrounding the role of epidural adhesions in radicular pain. Since it was first described in patients with failed back surgery syndrome, percutaneous adhesiolysis has been used to lyse epidural scars [7], and treat radicular pain in various diseases [13,14]. Conventional epidural adhesiolysis was performed via a caudal approach, and combinations of the following three methods are purportedly used: 1) volumetric: injection of a large volume of normal saline [7,14], 2) mechanical: the use of a specially designed catheter [14,15,16], and 3) chemical: the use of hypertonic saline and hyaluronidase [13,17,18]. Of these methods, we focused on volumetric adhesiolysis because it can be easily incorporated into a transforaminal approach without additional instrumentation or an increased risk of adverse reactions. Thus, 5 ml of saline was forcefully injected within 1-2 seconds, followed by the main steroid-containing injectate.
Although primary outcomes favored the DV group over the DO group, there was no statistically significant difference between the two groups. Our explanation of these findings was that the forceful injection of 5 ml normal saline was not enough to lyse adhesions effectively and make a significant clinical difference. For conventional epidural adhesiolysis, high-volume irrigation (60-80 ml of fluid) is typically performed via a caudal approach with or without catheterization [5,8]. There are few reports about transforaminal epidural adhesiolysis [13,16,18], and no study is available on a volumetric method alone. A higher volume of saline irrigation would be necessary for a more effective transforaminal approach, and conscious sedation with hypnotics and analgesics may be helpful considering severe paresthesias or radiating pain during a forceful injection.
Our study has several limitations. First, it included a small number of patients and was not a randomized controlled study. Second, no long-term follow-up was conducted. There is some evidence of differences between short-term and long-term effects of this type of treatment [19]. Finally, the degree of epidural adhesion may vary according to the disease entity causing radicular pain. It is also possible that the effects of adhesiolysis are time-dependent, as adhesion plays a less important role in acute pain than it does in chronic pain.
In conclusion, a forceful saline injection did not have a significant effect during the treatment of radicular pain. Further studies with greater volumes and with additional techniques would offer a more conclusive perspective.
Go to : 
References
1. Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: a comprehensive, evidence-based review. Reg Anesth Pain Med. 2013; 38:175–200. PMID: 23598728.
2. Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013; 16:S49–S283. PMID: 23615883.
3. Koc Z, Ozcakir S, Sivrioglu K, Gurbet A, Kucukoglu S. Effectiveness of physical therapy and epidural steroid injections in lumbar spinal stenosis. Spine (Phila Pa 1976). 2009; 34:985–989. PMID: 19404172.

4. Luijsterburg PA, Verhagen AP, Ostelo RW, van Os TA, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J. 2007; 16:881–899. PMID: 17415595.

5. Rabinovitch DL, Peliowski A, Furlan AD. Influence of lumbar epidural injection volume on pain relief for radicular leg pain and/or low back pain. Spine J. 2009; 9:509–517. PMID: 19398387.

6. Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Fluoroscopic caudal epidural injections with or without steroids in managing pain of lumbar spinal stenosis: one-year results of randomized, double-blind, active-controlled trial. J Spinal Disord Tech. 2012; 25:226–234. PMID: 22652990.

7. Racz GB, Day MR, Heavner JE, Scott J. Lysis of epidural adhesions: the Racztechnique. In : Waldman SD, editor. Pain management. 2nd ed. Philadelphia (PA): Elsevier Saunders;2011. p. 1258–1272.
8. Chun-jing H, Hao-xiong N, jia-xiang N. The application of percutaneous lysis of epidural adhesions in patients with failed back surgery syndrome. Acta Cir Bras. 2012; 27:357–362. PMID: 22534813.

9. Lee JH, Moon J, Lee SH. Comparison of effectiveness according to different approaches of epidural steroid injection in lumbosacral herniated disk and spinal stenosis. J Back Musculoskelet Rehabil. 2009; 22:83–89. PMID: 20023335.

10. Benzon HT, Chew TL, McCarthy RJ, Benzon HA, Walega DR. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007; 106:331–338. PMID: 17264728.

11. Derby R, Lee SH, Date ES, Lee JH, Lee CH. Size and aggregation of corticosteroids used for epidural injections. Pain Med. 2008; 9:227–234. PMID: 18298706.

12. Furman MB, Mehta AR, Kim RE, Simon JI, Patel R, Lee TS, et al. Injectate volumes needed to reach specific landmarks in lumbar transforaminal epidural injections. PM R. 2010; 2:625–635. PMID: 20659718.

13. Park CH, Lee SH. Effectiveness of percutaneous transforaminal adhesiolysis in patients with lumbar neuroforaminal spinal stenosis. Pain Physician. 2013; 16:E37–E43. PMID: 23340543.
14. Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis using Navicath for the management of chronic pain due to lumbosacral disc herniation. Pain Physician. 2012; 15:213–221. PMID: 22622905.
15. Jo DH, Yang HJ. The survey of the patient received the epiduroscopic laser neural decompression. Korean J Pain. 2013; 26:27–31. PMID: 23342204.

16. Kim SH, Koh WU, Park SJ, Choi WJ, Suh JH, Leem JG, et al. Clinical experiences of transforaminal balloon decompression for patients with spinal stenosis. Korean J Pain. 2012; 25:55–59. PMID: 22259719.

17. Heavner JE, Racz GB, Raj P. Percutaneous epidural neuroplasty: prospective evaluation of 0.9% NaCl versus 10% NaCl with or without hyaluronidase. Reg Anesth Pain Med. 1999; 24:202–207. PMID: 10338168.

18. Koh WU, Choi SS, Park SY, Joo EY, Kim SH, Lee JD, et al. Transforaminal hypertonic saline for the treatment of lumbar lateral canal stenosis: a double-blinded, randomized, active-control trial. Pain Physician. 2013; 16:197–211. PMID: 23703407.
19. El-Yahchouchi C, Geske JR, Carter RE, Diehn FE, Wald JT, Murthy NS, et al. The noninferiority of the nonparticulate steroid dexamethasone vs the particulate steroids beta-methasone and triamcinolone in lumbar transforaminal epidural steroid injections. Pain Med. 2013; 14:1650–1657. PMID: 23899304.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download