This article has been
cited by other articles in ScienceCentral.
Abstract
Background
To evaluate the results of conventional radiofrequency thermorhizotomy (CRT) for trigeminal neuralgia (TN) in patients with failed medical management.
Methods
Patients with Trigeminal neuralgia who were referred to us for 'limited intervention' during the time frame July-2011 to Jan-2013 were enrolled for this study. CRT was administered by the Sweet technique. Pain relief was evaluated by the principle investigator.
Results
Eighteen patients were enrolled and completed a mean follow-up of 18.0 months. Pain relief was observed in 14 of 18 (77.8%) patients on the post-operative day, 14 of 18 (77.8%) at 1-month follow-up, 14 of 17 (82.4%) at 3-months follow-up, 12 of 15 (80%) at 6-months follow-up, 7 of 11 (63.6%) at 1-year follow-up and 2 of 6 (33.3%) 1.5 years of follow-up. Four patients required a repeat cycle of CRT; two at six months of follow-up and two at one year of follow-up. One patient was transferred for surgical intervention at six months of follow-up. Side-effects included facial hypoesthesia (n = 6); nausea/vomiting (n = 2), diminished corneal reflex (n = 13) and difficulty in chewing (n = 11). Severity of adverse effects gradually diminished and none of the patients who are beyond 6 months of follow-up have any functional limitation.
Conclusions
CRT is an effective method of pain relief for patients with Trigeminal neuralgia. Successful outcome (excellent or good) can be expected in 66.7% of patients after first cycle of CRF. The incidence and severity of adverse effects is less and the procedure is better tolerated by the patients.
Go to :

Keywords: radiofrequency, treatment, trigeminal ganglion, trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) or tic douloureux is a chronic, neuropathic-pain disorder characterized by sporadic episodes of extreme, sudden-onset, burning/ stabbing unilateral facial pain lasting for a few seconds to minutes. The pain is incapacitating physically and mentally and confined within the territorial distribution of the trigeminal nerve.
A vast array of treatment modalities have been described although none of them has proved to be uniformly effective. The available options include medical management (carbamazepine is the drug of choice, oxcarbazepine, amitriptyline, gabapentin and baclofen), microvascular surgical decompression or gamma knife surgery. Local ablation of the peripheral nerve or Gasser's ganglion (with alcohol or phenol) and wide sectioning of the sensory roots have been largely abandoned.
The use of conventional or pulsed radiofrequency thermorhizotomy is relatively new and has been shown to be significantly effective in the relatively few studies available.
Go to :

MATERIALS AND METHODS
This is a prospective study conducted in the Department of Anesthesiology at the Banaras Hindu University over a period of one year nine months (July 2011-April 2013). Eighteen patients satisfying the following criteria were included in this study.
Primary TN (ICHD-II) for more than two years.
Failed medical management.
A pain Visual Analog Scale (VAS) score of more than 7/10 with a poor quality of life because of the pain.
Absence of concomitant disorders capable of accounting for the symptoms.
No organic disease on brain MRI.There was no involvement of any artery and vein.
Those patients who refused to participate in the study were excluded. Written informed consent was obtained from all patients prior to participation in this study. CRT was administered by the technique as described [
1]. Pain relief was evaluated by the principle investigator on the day of the procedure and during follow-up at 1 month, 3 months, 6 months and at 6 monthly intervals thereafter. Criteria for assessing pain relief were:
Subjective assessment: Pain relief as perceived by the patient (> 80% relief was graded as excellent, 50-80% pain relief as satisfactory and < 50% was graded as less than satisfactory).
Objective assessment: Reductionof 60% in the dose of analgesic medications.
Final outcome was divided into five categories as described by Park et al. [2]: 'Excellent' for those patients who were pain free and off pain medications, 'Good' for those who are in occasional pain controlled with or without medication, 'Poor' for those complaining of severe dysesthesia, not adequately controlled with medication, 'Failure' for patients with no post-surgical pain relief at one month and 'Recurrence' for those who experience the return of symptoms after a remission.
1. Techniques of CRT
CRT was performed percutaneously by the technique described by Sweet and Wepsic [
1] in 1974.
2. Requisites
Sterile drapes, 5% povidone iodine lotion, spirit, 18 guage intravenous canula, patient monitors, sand bag, neck roll, C-arm, injection glycopyrollate, injection ondansetron, injection fentanyl, injection propofol, injection hydrocortisone, injection lidocaine, 22-G Premium SMK canula/needle, radiofrequency generator (Baylis, Canada),and iohexol.
3. Procedure
Antiseptic mouthwash was advised a day prior to surgery. Procedure was performed in the operation theatre in a sterile environment. A wide bore intra-venous canula was secured and continuous hemo-dynamic monitoring established (including electrocardiography, blood pressure and pulse oximetry). The patient was positioned supine and the head was extended slightly with a sand-bag under the upper shoulders and a roll under the neck. The C-arm is set up and adjusted to provide a submental position and optimal visualization of the foramen ovale. Usually, this is possible by tilting the C-arm towards the affected side by 5-10 degrees. The patient is pre-medicated with glycopyrollate (0.01 mg/kg body weight, intravenous), ondansetron (4 mg, intravenous) and fentanyl (1-2 µg/kg body weight, intravenous).
The needle entry-point is marked 2-3 cm from the corner of the mouth and infiltrated with 1% lignocaine for local anesthesia. The direction of needle advancement is towards the ipsilateral pupil. However, while advancing the needle, it must be remembered to keep a finger in the oral cavity to detect any breach of the oral cavity. In case a breech is detected, it is advised to change the needle to reduce the incidence of infectious complications. The entry of the needle-tip into the Meckel's cavity inside the foramen ovale and the depth of penetration are confirmed with the C-arm. The needle is advanced till it overshoots the angle between the petrosal ridge of the temporal bone and the clivus.
Sensory stimulation is carried out at 50 Hz. The definitive position of the electrode was verified by inducing paresthesia with sensory stimulation between 0.05-0.3 Volts in the affecting territory. At this point, the patient is sedated with propofol (0.75 mg/kg body weight, intravenous). The needle tip temperature was maintained at 65 degree centigrade for 60 seconds, 70 degrees for 60 seconds and 75 degrees for 60 seconds to effect the thermorhizotomy of the trigeminal ganglion. After the procedure, 20 mg hydrocortisone with 1 ml lidocaine was injected locally.
Go to :

RESULTS
Eighteen patients were managed with CRT; the cohort included 7 male and 11 female patients. Age varied from 29-69 years (Mean 49.1 years). The territorial involvement included mandibular nerve (n = 5), maxillary nerve (n = 5), both mandibular and maxillary nerves (n = 7) and orbital nerve (n = 1). Right side (n = 11) was more frequently involved than the left (n = 7); none of the cases in this cohort had bilateral involvement.
All patients included in this study showed unsatisfactory response to carbamazepine (mean dose 1045 mg/day, range 600-1800 mg/day) or amitriptyline (mean dose 22.5 mg/day, range 10-30 mg/day) in maximal doses. Additional drugs tried for pain relief included phenytoin (n = 6, mean 245 mg/day, range 100-400 mg/day), gabapentin and baclofen (dosage details not available). Verbal Analogue Score (VAS) prior to initiation of therapy varied from 6-9 out of 10 (mean 6.7), after maximal medical management from 3-6 (mean 4.8) with a mean reduction in VAS by 2.9. Adverse effects limiting the role of medical management included dryness of mouth (n = 18), and blurred vision (n = 15), excessive drowsiness (n = 14), psychosis (n = 7), abnormal body movements (n = 6) and giddiness (n = 3).
Patients were followed up for a period ranging between 1 month and 18 months (Mean 8.7 months). On subjective assessment (
Table 1), pain relief (excellent or satisfactory) was observed in 14 of 18 (77.8%) patients on the post-operative day, 14 of 18 (77.8%) at 1-month follow-up, 14 of 17 (82.4%) at 3-months follow-up, 12 of 15 (80%) at 6-months follow-up, 7 of 11 (63.6%) at 1-year follow-up and 2 of 6 (33.33%) 1.5 years of follow-up. At 6-months of follow-up, two patients with no response were given a repeat cycle of CRF and another one was transferred for surgical intervention (did not consent for repeat CRF). Similarly, two more patients who experienced satisfactory pain relief at 6-months follow-up and subsequently pain relief was unsatisfactory at 1-year of follow-up were administered a repeat cycle of CRF. However, for the purpose of statistical analysis, these patients have been counted as no pain relief on subsequent follow-ups rather than excluding them from statistics. 2 of 4 (50%) had satisfactory pain relief after repeat cycle of CRF which was sustained till 3-months of follow-up (they continue to be in follow-up). The other two patients had to be transferred for surgical intervention because therewas no effect of treatment and they would not want to have repeat CRF. The objective assessment was in congruence with the subjective assessment (
Table 1). Final outcome after the first cycle of CRF was graded as excellent in 6 of 18 (33.3%) patients, good in 6 of 18 (33.3%), poor in 1 of 18 (5.6%), failed in 2 of 18 (11.1%) and recurrence in 3 of 18 (16.7%) patients. Successful outcome (excellent or good) can be expected in 66.7% of patients after first cycle of CRF.
Table 1
Results of Conventional Radiofrequency Thermorhizotomy for Trigeminal Neuralgia
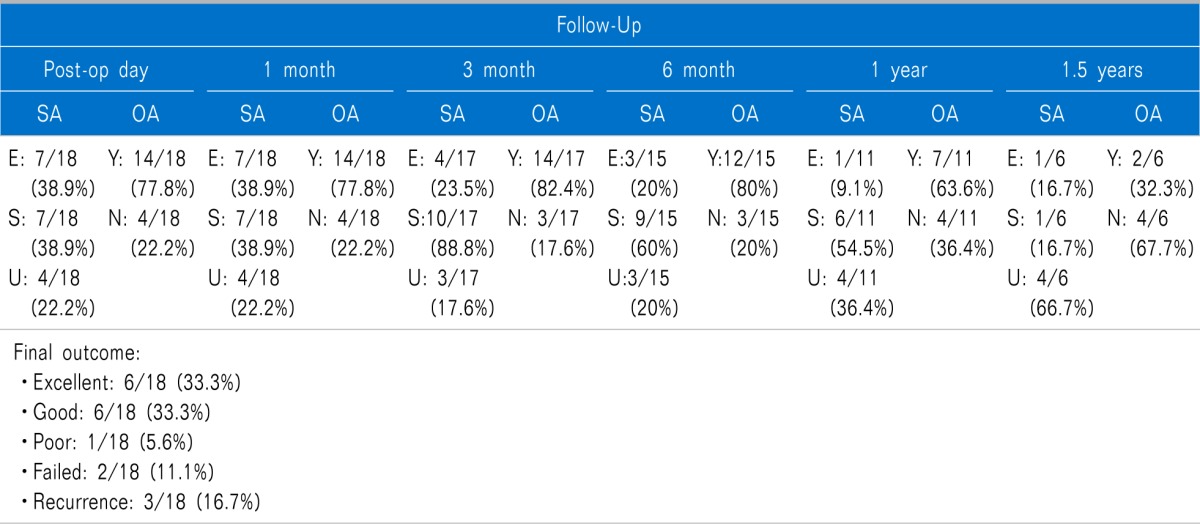

Side-effects included facial hypoesthesia (n = 6); nausea/vomiting (n = 2), diminished corneal reflex (n = 13) and difficulty in chewing (n = 11). Severity of adverse effects gradually diminished and none of the patients who are beyond 6 months of follow-up have any functional limitation.
Go to :

DISCUSSION
The International Headache Societyhas defined TN (ICHD-II) as "unilateral disorder characterized by brief electric shock-like pains, abrupt in onset and termination and limited to the distribution of one or more divisions of the trigeminal nerve". The pain imposes a substantial burden on these patients; they may not be able to eat or even speak during severe attacks. Some patients also complaints of an overwhelming fear of 'return of pain' in between attacks [
3]. The mechanisms associated with the development of this persistent pain [
4,
5,
6] are not well understood but concomitant background pain is associated with poor medical and surgical outcome.
The availability of a huge variety of pharmacological and surgical treatment options for TN votes in favor of uniform inefficiency of either of these modalities. Amongst the drugs available, carbamazepine and oxcarbazepine form the first-line therapy. Lamotrigine, baclofen and pimozide form the second-line and are usually given as add-on therapy. Phenytoin, clonazepam, gabapentin, topiramate, levetiracetam, valproate and tocainide are also beneficial. The problem with medical management is poor drug tolerance which is related to many factors. Progressively increasing dosages of carbamazepine are required to maintain efficacy which declines to approximately 50% due to autoinduction [
7]. Furthermore, due to age-related alterations in physiology & pharmacokinetics (reduced hepatic and renal function, reduced blood flow, less predictable drug protein binding and interactions with multiple other medications required due to co-morbidities) tolerance to drugs decreases with age while the incidence of TN increases with age [
8]. It has been estimated that approximately 6-10% of patients cannot tolerate CBZ [
9].
Surgical treatments are usually reserved for patients with debilitating pain refractory to an adequate trial of medical management. Percutaneous procedures on the Gasserian ganglion, gamma knife and microvascular decompression have been described. Surgery for TN is either ablative and involves intentional destruction of the trigeminal nerve sensory function or non-destructive, where the trigeminal nerve is decompressed preserving its normal function.But the morbidity and mortality associated to surgery specifically in this age group. Gasserian ganglion percutaneous techniques are all destructive and include radiofrequency thermorhizotomy, balloon compression (BC) and percutaneous glycerol rhizolysis (PGR).
Radiofrequency thermorhizotomyafter its initial description [
10] and has stood the test of time. However, there have been two major modifications in the technique. White and Sweet [
11] described the use of short-acting anesthetic agent, electrical stimulation, a reliable radiofrequency current for producing the lesion and monitoring the tip of electrode for temperature. The use of a curved tip-electrode and image-guided fluoroscopy for cannulation of the foramen ovale was suggested by van Loveren et al. [
12] and Tew et al. [
13]. Radiofrequency thermorhizotomy is based on the differential thermocoagulation property [
14] of trigeminal rootlets. The compound action potentials of A-δ and C fibers (nociceptive fibers) in a nerve are blocked at a lower temperature than those of A-α and A-β fibres that carry tactile sensations. The overall sensory input to the demyelinated peripheral site of ephaptic transmission is reduced [
15].
In this study, we have studied the pattern of pain distribution in patients with TN. Mandibular branch of trigeminal nerve was involved in two-third of the patients (isolated involvement in 27.8%), maxillary branch also in two-third (isolated involvement in 27.8%) while the orbital branch was involved in only one of eighteen (5.6%) patients. Right side (61.1%) is more frequently involved that the left. The subjective (patients' input) and objective (reduction in dose of analgesics) assessments of pain relief were in absolute correlation. Pain relief was reported in 14 of 18 (77.8%) patients on the post-operative day, 14 of 18 (77.8%) at 1-month follow-up, 14 of 17 (82.4%) at 3-months follow-up, 12 of 15 (80%) at 6-months follow-up, 7 of 11 (63.6%) at 1-year follow-up and 2 of 6 (33.3%) 1.5 years of follow-up. Final outcome after the first cycle of CRF was graded as excellent in one-third of the patients and good in another one-third. Failure was observed in 11.1% and recurrence in 16.7% of the patients. Successful outcome (excellent or good) can be expected in 66.7% of patients after first cycle of CRF. Satisfactory pain relief was observed in half of those patients who required a second cycle of CRF.
Liu et al. [
16] probably published the largest series on radiofrequency thermocoagulation in primary TN (ICHD-II) to analyze its clinical efficacy and discuss the method, skill of radiofrequency thermocoagulation its complications. In a cohort of 648 patients, the rate of pain control was 98.3% via foramen infraorbital approach, 91.0% via lateral approach and 95.5% via former approach. The overall response rate was 96.0%. 395 patients were followed up from 6 months to 2 years. The recurrent rate within one year was 9.6%, 20.5% within two years. Good response was achieved after re-treatment with radiofrequency thermocoagulation in recurrent patients. They concluded that the clinical efficacy of radiofrequency thermocoagulation in the treatment of idiopathic TN is good and reliable. The procedure is simple, the indication is wide, and the complication is fewer. CT location can improve the accuracy of puncture, and reduce complications.
Erdine et al. [
17] compared pulsed radiofrequency with conventional radiofrequency in the treatment of TN on the basis of the following evaluation criteria: pain intensity using a Visual Analogue Scale (VAS), patient satisfaction using a Patient Satisfaction Scale (PSS), additional pharmacological treatment, side effects, and complications related to the technique. They demonstrated that unlike conventional radiofrequency, pulsed radiofrequency is not an effective method of pain treatment for idiopathic TN. Chua et al. [
18], however, demonstrated excellent pain relief (> 80% pain relief) at 2, 6, and 12 months were 73.5% (25/34), 61.8% (21/34), and 55.9% (19/34), respectively with pulsed TN.
Kim et al. [
19] showed that although the conventional method had more complications than pulsed radiofrequency thermorhizotomy in patients with dental procedure-induced TN; however, most of these complications were minor and transient, and the patient satisfaction rate was higher with the conventional method.
Fang et al. [
20] studied the role of 3D CT-Guided pulsed radiofrequency treatment for TN and found that it is safer than the conventional method though the results are not superior to the conventional technique.
Chen et al. [
21] suggested the use of electromagnetic navigation (EMN)-guided radiofrequecythermocoagulation (RFT) in these patients. They demonstrated the effectiveness and accuracy for radiofrequency thermocoagulation in three patients with primary TN (ICHD-II) in an attempt to make the process more simple and accurate.
In conclusion, conventional radiofrequency thermorhizotomy is a safe and effective means for treatment of TN. Successful outcome (excellent or good) can be expected in 66.7% of patients after first cycle of CRF. A repeat procedure may be performed in those who are either not benefitted after the first cycle or who develop a recurrence. Repeat procedure does not pose a significant problem and is associated with minimal risk. However, in view of the small cohort of cases involved in this study, a larger study is highly recommended.
Go to :

ACKNOWLEDGEMENTS
Dr. Prabudh Goel, Asst. Professor in Pediatric Surgery in the King George's Medical University, Lucknow for writing this manuscript on behalf of the authors.
Go to :

References
1. Sweet WH, Wepsic JG. Controlled thermocoagulation of trigeminal ganglion and rootlets for differential destruction of pain fibers. 1. Trigeminal neuralgia. J Neurosurg. 1974; 40:143–156. PMID:
4587949.

2. Park SS, Lee MK, Kim JW, Jung JY, Kim IS, Ghang CG. Percutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia : experience in 50 patients. J Korean Neurosurg Soc. 2008; 43:186–189. PMID:
19096641.

3. Cheshire WP. Trigeminal neuralgia feigns the terrorist. Cephalalgia. 2003; 23:230. PMID:
12662192.

4. Obermann M, Yoon MS, Sensen K, Maschke M, Diener HC, Katsarava Z. Efficacy of pregabalin in the treatment of trigeminal neuralgia. Cephalalgia. 2008; 28:174–181. PMID:
18039340.

5. Sandell T, Eide PK. Effect of microvascular decompression in trigeminal neuralgia patients with or without constant pain. Neurosurgery. 2008; 63:93–99. PMID:
18728573.

6. Szapiro J Jr, Sindou M, Szapiro J. Prognostic factors in microvascular decompression for trigeminal neuralgia. Neurosurgery. 1985; 17:920–929. PMID:
4080125.

7. Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966; 29:265–267. PMID:
5327969.

8. Khan OA. Gabapentin relieves trigeminal neuralgia in multiple sclerosis patients. Neurology. 1998; 51:611–614. PMID:
9710050.

9. Taylor JC, Brauer S, Espir ML. Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad Med J. 1981; 57:16–18. PMID:
7279817.

10. Bovaira M, Peñarrocha M, Peñarrocha M, Calvo A. Conventional radiofrequency treatment in five patients with trigeminal neuralgia. Med Oral Patol Oral Cir Bucal. 2013; 18:e76–e80. PMID:
23229234.

11. White JC, Sweet WH. Pain and the neurosurgeon. Springfield (IL): Charles C. Thomas;1969.
12. van Loveren H, Tew JM Jr, Keller JT, Nurre MA. A 10-year experience in the treatment of trigeminal neuralgia. Comparison of percutaneous stereotaxic rhizotomy and posterior fossa exploration. J Neurosurg. 1982; 57:757–764. PMID:
6754883.
13. Tew JM Jr, Keller JT, Williams DS. Application of stereotactic principles to the treatment of trigeminal neuralgia. Appl Neurophysiol. 1978; 41:146–156. PMID:
365093.

14. Letcher FS, Goldring S. The effect of radiofrequency current and heat on peripheral nerve action potential in the cat. J Neurosurg. 1968; 29:42–47. PMID:
5674091.

15. Brown JA. Percutaneous techniques. In : Winn HR, editor. Youmans neurological surgery. 5th ed. Philadelphia (PA): Saunders;2003. p. 2996–2999.
16. Liu C, Zhou ZG, Yuan CY. Treatment of primary trigeminal neuralgia with radiofrequency thermocoagulation: report of 648 consecutive cases. Shanghai Kou Qiang Yi Xue. 2012; 21:466–469. PMID:
23135127.
17. Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007; 11:309–313. PMID:
16762570.

18. Chua NH, Halim W, Beems T, Vissers KC. Pulsed radiofrequency treatment for trigeminal neuralgia. Anesth Pain Med. 2012; 1:257–261. PMID:
24904811.

19. Kim JH, Yu HY, Park SY, Lee SC, Kim YC. Pulsed and conventional radiofrequency treatment: which is effective for dental procedure-related symptomatic trigeminal neuralgia? Pain Med. 2013; 14:430–435. PMID:
23432997.

20. Fang L, Ying S, Tao W, Lan M, Xiaotong Y, Nan J. 3D CT-guided pulsed radiofrequency treatment for trigeminal neuralgia. Pain Pract. 2014; 14:16–21. PMID:
23433058.

21. Chen MJ, Gu LX, Zhang WJ, Yang C, Dong MJ. Electromagnetic navigation-guided radiofrequency thermocoagulation in trigeminal neuralgia: technical note with three case reports. J Neurol Surg A Cent Eur Neurosurg. 2013; 74:251–257. PMID:
23307309.

Go to :






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download