INTRODUCTION
Bupivacaine, a long-acting aminoamide local anesthetic that exists in a mixture of two forms (levorotatory and dextrorotatory) and has a relatively high risk of cardiotoxicity among local anesthetics, is widely used for labor analgesia [
1]. Bupivacaine produces vasorelaxation in isolated vessels precontracted with norepinephrine and attenuates contraction induced by thromboxane A
2 or norepinephrine [
2,
3]. In isolated endothelium-denuded aortas with resting tension, levobupivacaine produces vasoconstriction at lower doses and vasodilation at higher doses, suggesting that the effect of levobupivacaine on vascular tone is concentration-dependent [
4,
5,
6,
7,
8,
9]. A toxic dose of bupivacaine produces vasodilation in endothelium-denuded aortas precontracted with KCl [
10]. Local anesthetic systemic toxicity induced by bupivacaine or levobupivacaine produces severe vascular collapse mediated by vasodilation [
4,
5,
6,
7,
8,
9,
10]. Hyperpolarization in the membrane potential of vascular smooth muscle induced by the activation of various potassium channels, including voltage-dependent, calcium-activated, inward-rectifying, and adenosine triphosphate-sensitive potassium channels, produces vasodilation via the inhibition of calcium influx [
11]. In addition, etomidate and alfentanil attenuate phenylephrine-induced contraction via an inhibitory effect on voltage-operated calcium channels [
12,
13]. Rho-kinase and protein kinase C are involved in the increased calcium sensitization, which lead to local anesthetic-induced vasoconstriction, whereas decreased calcium sensitization attenuates local anesthetic-induced vasoconstriction, which may be involved in vasodilation [
6,
14,
15]. However, the cellular mechanism responsible for bupivacaine-induced relaxation in precontracted isolated rat aortas remains unknown. Therefore, the goal of this
in vitro study was to investigate the cellular mechanism associated with bupivacaine-induced vasodilation in isolated endothelium-denuded rat aortas precontracted with phenylephrine.
Go to :

MATERIALS AND METHODS
All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences.
1. Preparation of aortic rings for tension measurement
Male Sprague-Dawley rats (n = 35) weighing 250-300 g were anesthetized with intramuscular administration of Zoletil 50 (tiletamine HCl 125 mg and zolazepam 125 mg/5 ml; 15 mg/kg; Virbac Laboratories, Carros, France). The descending thoracic aorta of each rat was dissected free, and the surrounding connective tissues and fat were removed under microscopic guidance in a Krebs solution bath of the following composition: 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO
4, 1.2 mM KH
2PO
4, 2.4 mM CaCl
2, 25 mM NaHCO
3, and 11 mM glucose. Each aorta was cut into 2.5-mm rings, suspended on Grass isometric transducers (FT-03, Grass Instrument, Quincy, MA, USA) under a 3.0-g resting tension in a 10-mL Krebs bath at 37℃, and aerated continuously with 95% O
2 and 5% CO
2 to maintain the pH value within the range of 7.35-7.45. The rings were equilibrated at a 3.0-g resting tension for 120 min, and the bath solution was changed every 30 min. The endothelium was removed from the aortic rings by inserting a 25-gauge needle tip into the lumen of the rings and gently rubbing the ring for a few seconds. As soon as the phenylephrine (10
-8 M)-induced contraction had stabilized, endothelial removal was confirmed by the observation of less than 10% relaxation in response to acetylcholine (10
-5 M). After washing out the phenylephrine from the organ bath and allowing for the return of isometric tension to the baseline resting tension, the main experiment was performed as described in the experimental protocols. The contractile response induced by isotonic 60 mM KCl was measured in an endothelium-denuded aortic ring used for phenylephrine concentration-response curves, and it was used as a reference value to express the magnitude of the contractile response induced by the cumulative addition of phenylephrine. In the main experiments involving only the endothelium-denuded aortas, the Krebs solution also contained the nitric oxide synthase inhibitor N
W-nitro-L-arginine methyl ester (L-NAME, 10
-4 M) to prevent the release of endogenous nitric oxide from any residual endothelium [
6,
7,
9].
2. Experimental protocol
First, the effects of various potassium channel inhibitors on bupivacaine concentration-response curves in the endothelium-denuded aortas precontracted with phenylephrine were assessed. Glibenclamide (10
-5 M), an adenosine triphosphate-sensitive potassium channel inhibitor, or iberiotoxin (10
-7 M), a large conductance calcium-activated potassium channel inhibitor, were added directly to the organ bath for 20 min before the addition of phenylephrine (10
-7 M). Because pretreatment with the voltage-dependent potassium channel inhibitor 4-aminopyridine (2 × 10
-3 M) or the inward rectifying potassium channel inhibitor barium chloride (3 × 10
-5 M) shifted upward the baseline resting tension for the incubation period before the addition of phenylephrine, we added 4-aminopyridine (2 × 10
-3 M) or barium chloride (3 × 10
-5 M) into the organ bath about 3 min after the addition of phenylephrine (10
-7 M). We then waited for about 30 min so that the phenylephrine-induced contraction could plateau. After the phenylephrine (10
-7 M)-induced contraction had stabilized, incremental concentrations (5.36 × 10
-7 to 9.51 × 10
-4 M) of bupivacaine were added to the organ bath to generate bupivacaine concentration-response curves. The effect of various potassium channel inhibitors on the bupivacaine concentration (5.36 × 10
-7 to 9.51 × 10
-4 M)-response curves was assessed by comparing the bupivacaine-induced vasorelaxant response in the presence or absence of each potassium channel inhibitor. Concentrations of various potassium channel inhibitors were chosen on the basis of the concentrations used in previous experiments similar to this experiment [
11,
16,
17,
18].
Second, the effect of voltage-operated calcium channel inhibitors on the bupivacaine concentration-response curves in endothelium-denuded aortas precontracted with phenylephrine (3 × 10
-6 M) was assessed. The voltage-operated calcium channel inhibitors nifedipine (10
-9, 10
-8 and 10
-7 M) and verapamil (10
-6 and 10
-5 M) were added to the organ bath for 20 min before the addition of phenylephrine (3 × 10
-6 M) [
7]. After the phenylephrine (3 × 10
-6 M)-induced contraction had stabilized, incremental concentrations of bupivacaine were added to the organ bath to generate bupivacaine concentration-response curves. The effects of the voltage-operated calcium channel inhibitors on the bupivacaine concentration-response curves were assessed by comparing the bupivacaine-induced vasorelaxant response in the presence or absence of either verapamil or nifedipine.
Third, we assessed the bupivacaine concentration-response curves in endothelium-denuded aortas precontracted with phenylephrine (3 × 10-6 M) or 100 mM KCl to examine whether the magnitude of the relaxant response induced by bupivacaine is dependent on the contractile agonist (phenylephrine or KCl) used for precontraction of the endothelium-denuded aortas before the cumulative addition of bupivacaine. After phenylephrine (3 × 10-6 M) or 100 mM KCl had produced a stable and sustained contraction, bupivacaine was added directly to the organ bath to produce cumulative bupivacaine concentration-response curves.
Finally, the effects of verapamil (10-5 and 10-6 M) on the phenylephrine concentration (10-9 to 10-5 M)-response curves were assessed to examine whether phenylephrine-induced contraction involves the activation of voltageoperated calcium channels. Verapamil (10-5 and 10-6 M) was added to the organ bath for 20 min before the addition of phenylephrine. Incremental concentrations of phenylephrine (10-9 to 10-5 M) were cumulatively added to the organ bath to generate phenylephrine concentration-response curves in the presence or absence of verapamil.
3. Fura-2 loading and simultaneous measurement of intracellular calcium concentration [Ca2+]i and tension
[Ca
2+]
i was measured according to the method described by Shim et al. [
6] using the fluorescent Ca
2+ indicator fura-2. Muscle strips were exposed to the acetoxymethyl ester of fura-2 (fura-2/AM, 10 µM) in the presence of 0.02% cremophor EL for 5-6 h at room temperature. After loading, a muscle strip was washed with Krebs solution at 37℃ for 20 min to remove any uncleaved fura-2/AM and held horizontally in a temperature-controlled, 7 ml organ bath. One end of the muscle strip was connected to a force-displacement transducer (MLT050, AD Instruments, Colorado Springs, CO, USA) to monitor the muscle contraction. The muscle strip was illuminated alternately (48 Hz) at two excitation wavelengths (340 and 380 nm). The intensity of 500 nm fluorescence (F340 and F380) was measured with a fluorometer (CAF-110, Jasco, Tokyo). The ratio of F340 to F380 (F340/F380) was calculated as an indicator of [Ca
2+]
i. The absolute Ca
2+ concentration was not calculated in this experiment because the dissociation constant of the fluorescence indicator for Ca
2+ in cytosol may be different from that obtained
in vitro [
19]. Therefore, the ratio (F340/F380) and tension obtained from either 3 × 10
-6 M phenylephrine or 100 mM KCl-stimulated aortic strips were taken as 100 and 100%, respectively. Isometric contractions and the ratio of F340/F380 were recorded with a PowerLab/400 using the chart program (AD instruments). Muscle strips were placed under an initial 3.0-g resting tension. All strips that came from the same animal were used in a different experimental protocol. When 100 mM KCl- or 3 × 10
-6 M phenylephrine-stimulated [Ca
2+]
i and contraction reached steady state levels, various bupivacaine contents (5.36 × 10
-6, 1.61 × 10
-5, 5.36 × 10
-5, 2.52 × 10
-4 and 9.51 × 10
-4 M) were added cumulatively.
4. Drugs
All drugs were of the highest purity available commercially. Verapamil, nifedipine, 4-aminopyridine, barium chloride, glibenclamide, acetylcholine, L-NAME, and phenylephrine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Bupivacaine was obtained from Reyon Pharmaceutical Co., Ltd. (Seoul, Korea). Iberiotoxin was obtained from Tocris Bioscience (Bristol, United Kingdom). Fura-2/AM was obtained from Molecular Probes (Eugene, OR, USA). All concentrations are expressed as the final molar concentration in the organ bath. Glibenclamide, nifedipine, and fura-2/AM were dissolved in dimethyl sulfoxide (DMSO) (final organ bath concentration: 0.1% DMSO). Unless stated otherwise, all drugs were dissolved in distilled water.
5. Data analysis
Values are expressed as the mean ± SD. N indicates the number of isolated descending thoracic aortic rings. Relaxant responses to bupivacaine in endothelium-denuded aortas precontracted with phenylephrine (10-7 or 3 × 10-6 M) or 100 mM KCl are expressed as the percentage of the baseline precontraction induced by phenylephrine (10-7 or 3 × 10-6 M) or 100 mM KCl. Contractile responses to phenylephrine in endothelium-denuded aortas with resting tension are expressed as the percentage of the maximum contractile response induced by isotonic 60 mM KCl. The relaxant response and [Ca2+]i decrease induced by bupivacaine are expressed as the percentage of the maximal contraction and [Ca2+]i induced by phenylephrine or KCl, respectively. The effects of potassium or calcium channel inhibitors on bupivacaine-induced concentration-response curves and phenylephrine-induced concentration-response curves were analyzed with two-way repeated measure analysis of variance followed by the Bonferroni post-test (Prism 5.0, GraphPad Software, San Diego, CA, USA). The effect of contractile agonists (phenylephrine or KCl) on bupivacaine-induced concentration-response curves was analyzed with two-way repeated measure analysis of variance followed by the Bonferroni post-test. The relaxant response and [Ca2+]i decrease with each concentration of bupivacaine were analyzed using repeated measures analysis of variance (ANOVA) followed by the Bonferroni post-test. Comparison between tension and [Ca2+]i induced by the cumulative addition of bupivacaine was performed using two-way repeated measure analysis of variance followed by the Bonferroni post-test. P values less than 0.05 were considered significant.
Go to :

RESULTS
Pretreatment with large conductance calcium-activated potassium channel inhibitor iberiotoxin (10
-7 M), voltagedependent potassium channel inhibitor 4-aminopyridine (2 × 10
-3 M), adenosine triphosphate-sensitive potassium channel inhibitor glibenclamide (10
-5 M), and inward rectifying potassium channel inhibitor barium chloride (3 × 10
-5 M) had no effect on bupivacaine-induced relaxation in isolated endothelium-denuded aortas precontracted with phenylephrine (10
-7 M) (
Fig. 1).
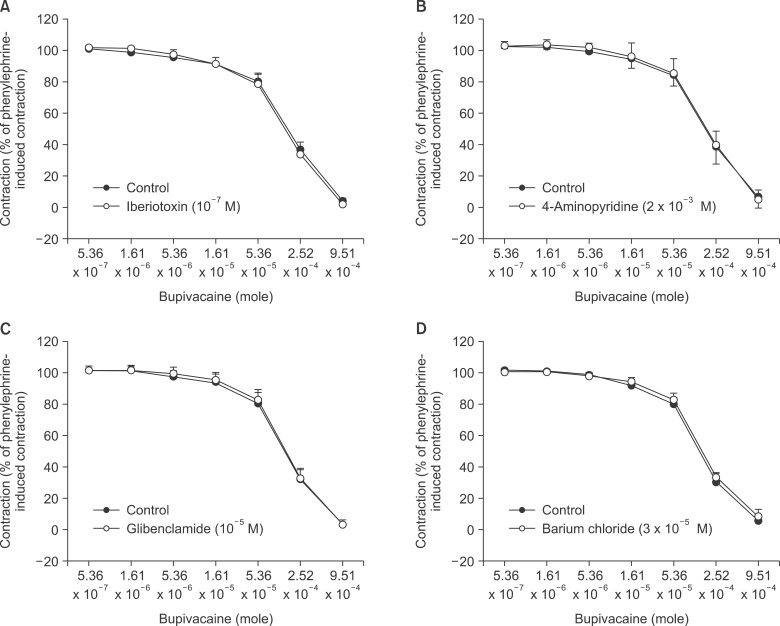 | Fig. 1The effects of iberiotoxin (10-7 M, A), 4-aminopyridine (2 × 10-3 M, B), glibenclamide (10-5 M, C), and barium chloride (3 × 10-5 M, D) on bupivacaine-induced concentration-response curves in isolated endothelium-denuded aortas precontracted with 10-7 M phenylephrine. Data are the means ± SD expressed as the percentage of the maximal contraction induced by phenylephrine (100% = 2.90 ± 0.97 g [n = 8] and 100% = 2.80 ± 0.66 g [n = 8] for endothelium-denuded rings with control and 10-7 M iberiotoxin, respectively, in A; 100% = 2.42 ± 0.53 g [n = 9] and 100% = 2.49 ± 0.55 g [n = 9] for endothelium-denuded rings with control and 2 × 10-3 M 4-aminopyridine, respectively, in B; 100% = 2.43 ± 0.67 g [n = 8] and 100% = 2.53 ± 0.34 g [n = 8] for endothelium-denuded rings with control and 10-5 M glibenclamide, respectively, in C; 100% = 3.20 ± 0.40 g [n = 6] and 100% = 3.09 ± 0.24 g [n = 6] for endothelium-denuded rings with control and 3 × 10-5 M barium chloride, respectively, in D). N indicates the number of isolated descending thoracic aortic rings. 
|
Verapamil (10
-6 and 10
-5 M) and nifedipine (10
-9, 10
-8, and 10
-7 M) attenuated bupivacaine-induced relaxation in endothelium-denuded aortas precontracted with 3 × 10
-6 M phenylephrine in a concentration-dependent manner (verapamil:
P < 0.001 versus control at 5.36 × 10
-5 and 2.52 × 10
-4 M bupivacaine; nifedipine:
P < 0.001 versus control at 2.52 × 10
-4 M bupivacaine) (
Fig. 2).
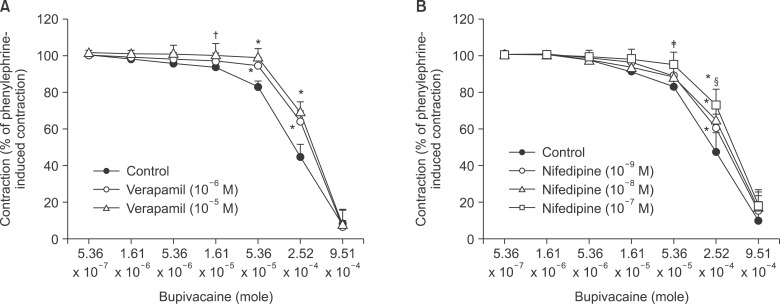 | Fig. 2The effects of verapamil (10-6 and 10-5 M; A) and nifedipine (10-9, 10-8 and 10-7 M; B) on bupivacaine-induced concentration-response curves in isolated endothelium-denuded aortas precontracted with 3 × 10-6 M phenylephrine. Data are the means ± SD expressed as the percentage of the maximal contraction induced by phenylephrine (100% = 2.42 ± 0.61 g [n = 6], 100% = 2.02 ± 0.41 g [n = 5], and 100% = 1.85 ± 0.38 g [n = 5] for endothelium-denuded rings with control, 10-6 M verapamil, and 10-5 M verapamil, respectively, in A; 100% = 2.81 ± 0.65 g [n = 8], 100% = 3.06 ± 0.45 g [n = 7], 100% = 3.01 ± 0.41 g [n = 6], and 100% = 2.50 ± 0.77 g [n = 6] for endothelium-denuded rings with control, 10-9 M nifedipine, 10-8 nifedipine, and 10-7 M nifedipine, respectively, in B). N indicates the number of isolated descending thoracic aortic rings. *P < 0.001, †P < 0.05, and ‡P < 0.01 versus control. §P < 0.01 versus 10-9 M nifedipine. 
|
The magnitude of bupivacaine-induced relaxation was higher in endothelium-denuded aortas precontracted with 100 mM KCl than in those precontracted with 3 × 10
-6 M phenylephrine (
P < 0.01 at 1.61 × 10
-5 to 2.52 × 10
-4 M bupivacaine) (
Fig. 3).
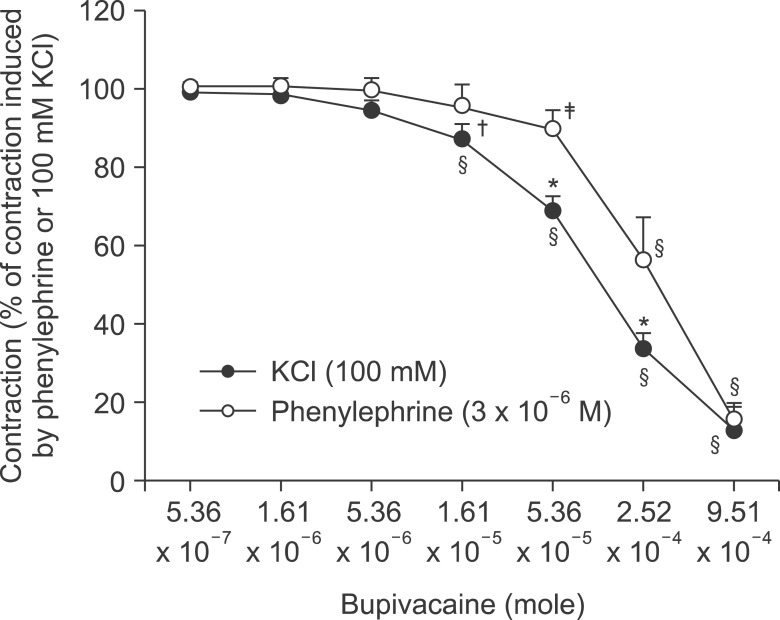 | Fig. 3Bupivacaine concentration-response curve in isolated endothelium-denuded aortas precontracted with 3 × 10-6 M phenylephrine or 100 mM KCl. Data are the means ± SD expressed as the percentage of the maximal contraction induced by the contractile agonist (100% = 2.85 ± 0.59 [n = 8] and 100% = 2.68 ± 0.34 g [n = 8] for endothelium-denuded rings with 3 × 10-6 M phenylephrine and 100 mM KCl, respectively). N indicates the number of isolated descending thoracic aortic rings. *P < 0.001 and †P < 0.01 versus 3 × 10-6 M phenylephrine. ‡P < 0.01 and §P < 0.001 versus 5.36 × 10-7 M bupivacaine. 
|
Verapamil (10
-5 and 10
-6 M) attenuated phenyl ephrine-induced contraction in a concentration-dependent manner (
P < 0.001 versus control at 10
-8 to 10
-5 M bupivacaine,
Fig. 4).
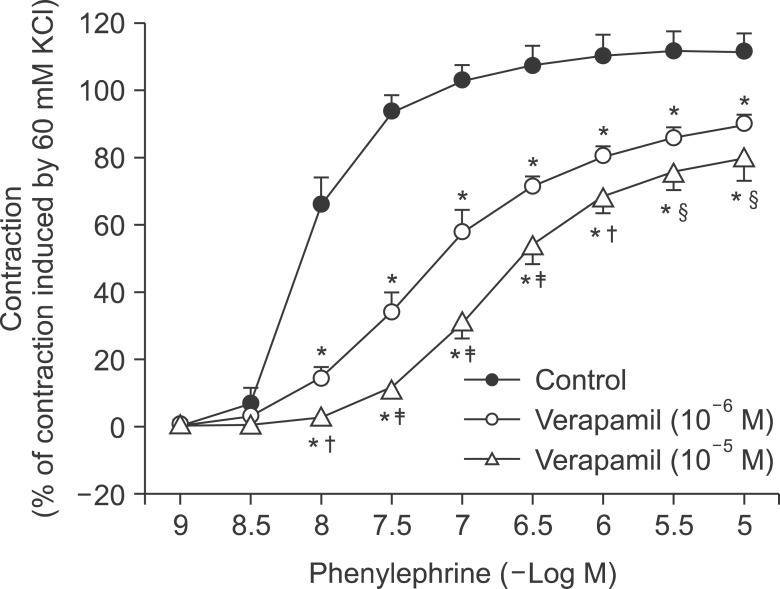 | Fig. 4The effects of verapamil (10-6 and 10-5 M) on phenylephrine concentration-response curves in isolated endothelium-denuded aortas. Data are the means ± SD expressed as the percentage of the maximal contraction induced by isotonic 60 mM KCl (100% = 2.33 ± 0.45 g [n = 5], 100% = 2.78 ± 0.08 g [n = 5], and 100% = 2.67 ± 0.48 g [n = 5] for endothelium-denuded rings with control, 10-6 M verapamil and 10-5 M verapamil, respectively). N indicates the number of isolated descending thoracic aortic rings. *P < 0.001 versus control. †P < 0.01, ‡P < 0.001, and §P < 0.05 versus 10-6 M verapamil. 
|
In aortas precontracted with phenylephrine, bupivacaine produced vasodilation (
P < 0.05 versus 3 × 10
-6 M phenylephrine) and decreased [Ca
2+]
i (
P < 0.001 versus 3 × 10
-6 M phenylephrine) (
Fig. 5). The magnitude of the bupivacaine-induced relaxation was higher than that of the bupivacaine-induced [Ca
2+]
i decrease in aortas precontracted with phenylephrine (
P < 0.001 at 2.52 × 10
-4 and 9.51 × 10
-4 M bupivacaine;
Fig. 5B). In aortas precontracted with KCl, bupivacaine produced vasodilation (
P < 0.05 versus 100 mM KCl) and decreased [Ca
2+]
i (
P < 0.01 versus 100 mM KCl) (
Fig. 6). The magnitude of the bupivacaine-induced relaxation was not significantly different from that of the bupivacaine-induced [Ca
2+]
i decrease in the aortas precontracted with KCl (
Fig. 6B).
 | Fig. 5(A) Effect of bupivacaine on phenylephrine (3 × 10-6 M)-stimulated intracellular calcium concentration ([Ca2+]i) (upper trace) and muscle tension (lower trace) in endothelium-denuded rat thoracic aortas. The [Ca2+]i of fura-2-loaded aortic strips was detected using a fluorometer and expressed as the ratio F340/F380. 100% represents the phenylephrine (3 × 10-6 M)-induced increases in both [Ca2+]i and muscle tension before the cumulative addition of bupivacaine. When the [Ca2+]i and muscle tension induced by phenylephrine reached a steady state level, various bupivacaine contents (5.36 × 10-6, 1.61 × 10-5, 5.36 × 10-5, 2.52 × 10-4 and 9.51 × 10-4 M) were cumulatively added. (B) Concentration-inhibition curve for bupivacaine in phenylephrine (3 × 10-6 M)-stimulated endothelium-denuded rat thoracic aortas. Various bupivacaine contents were cumulatively applied during the sustained increases in both [Ca2+]i and tension induced by phenylephrine (3 × 10-6 M). 100% represents the phenylephrine (3 × 10-6 M)-induced increase in both [Ca2+]i and muscle tension before the cumulative addition of bupivacaine. Each point represents the mean of 5 experiments, and SD is shown by vertical bars. *P < 0.05 and †P < 0.001 versus phenylephrine (3 × 10-6 M). ‡P < 0.001 versus F340/F380. 
|
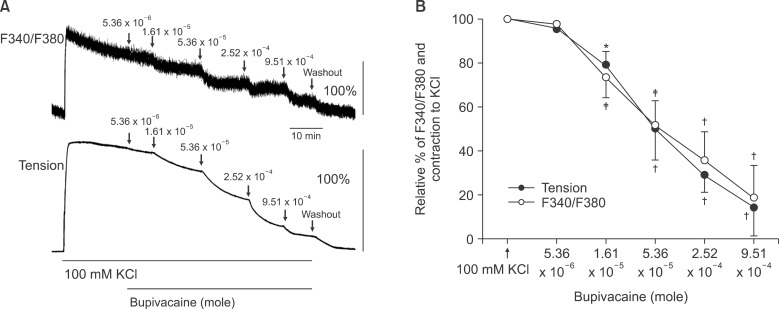 | Fig. 6(A) Effect of bupivacaine on high KCl (100 mM)-stimulated intracellular calcium concentration ([Ca2+]i) (upper trace) and muscle tension (lower trace) in endothelium-denuded rat thoracic aortas. The [Ca2+]i of fura-2-loaded aortic strips was detected using a fluorometer and expressed as the ratio F340/F380. 100% represents the 100 mM KCl-induced increases in both [Ca2+]i and muscle tension before the cumulative addition of bupivacaine. When the [Ca2+]i and muscle tension induced by high KCl reached a steady state level, various bupivacaine contents (5.36 × 10-6, 1.61 × 10-5, 5.36 × 10-5, 2.52 × 10-4 and 9.51 × 10-4 M) were cumulatively added. (B) Concentration-inhibition curve for bupivacaine in high KCl (100 mM)-stimulated endothelium-denuded rat thoracic aortas. Various bupivacaine contents were cumulatively applied during the sustained increases in both [Ca2+]i and tension induced by high KCl (100 mM). 100% represents 100 mM KCl-induced increase in both [Ca2+]i and muscle tension before the cumulative addition of bupivacaine. Each point represents the mean of 5 experiments, and SD is shown by vertical bars. *P < 0.05, †P < 0.001 and ‡P < 0.01 versus 100 mM KCl. 
|
Go to :

DISCUSSION
This study provides new evidence to suggest that bupivacaine-induced vasodilation in isolated rat aortas precontracted with phenylephrine appears to be associated with the inhibition of calcium sensitization. The major findings of this in vitro study are as follows: 1) the magnitude of the bupivacaine-induced relaxation was higher than that of the bupivacaine-induced [Ca2+]i decrease in aortas precontracted with phenylephrine, suggesting decreased calcium sensitization; 2) verapamil and nifedipine attenuated bupivacaine-induced relaxation; 3) the magnitude of the bupivacaine-induced relaxation was higher in 100 mM KCl-induced precontracted aortas than in phenylephrine-induced precontracted aortas; and 4) potassium channel inhibitors had no effect on bupivacaine-induced relaxation.
The stimulation of potassium channels leads to potassium efflux and subsequently induces membrane hyperpolarization, which promotes vasorelaxation via inhibition of calcium influx through voltage-operated calcium channels [
11,
20]. The activation of potassium channels, including large conductance calcium-activated and voltagedependent potassium channels, by membrane depolarization induced by a contractile agonist limits vasoconstriction as a negative feedback mechanism [
20]. Pretreatment with iberiotoxin or 4-aminopyridine had no effect on the bupivacaine-induced relaxation in isolated endothelium-denuded aortas precontracted with phenylephrine, suggesting that bupivacaine-induced relaxation does not involve the activation of large conductance calcium-activated and voltage-dependent potassium channels. In addition, glibenclamide and barium chloride had no effect on bupivacaine-induced relaxation, suggesting that bupivacaine-induced relaxation does not activate adenosine triphosphate-sensitive and inward rectifying potassium channels. However, the voltage-operated calcium channel inhibitors nifedipine and verapamil attenuated the bupivacaine-induced relaxation (
Fig. 2), suggesting an inhibitory effect of bupivacaine on voltage-operated calcium channels. In previous studies using isolated rat aortas with resting tension, the magnitude of vasodilation induced by the toxic dose (3 × 10
-4 M) of levobupivacaine was attenuated by verapamil or nifedipine, suggesting that toxic-dose levobupivacaine-induced vasodilation may involve the inhibition of voltage-operated calcium channels [
7]. In addition, a single toxic dose of levobupivacaine (3 × 10
-4 M) and bupivacaine (3 × 10
-4 M) produces relaxation in isolated endothelium-denuded aortas precontracted with 60 mM KCl [
4,
8,
10]. Furthermore, the toxic dose of levobupivacaine (10
-4 and 3 × 10
-4 M) inhibits 60 mM KCl-induced contraction [
7].
In the present study, the magnitude of the bupivacaine-induced relaxation was strongly dependent on that of the bupivacaine-induced [Ca
2+]
i decrease in aortas precontracted with KCl (
Fig. 6). Taking into consideration the previous reports and current results, bupivacaine (above 1.61 × 10
-5 M)-induced relaxation appears to be associated with the inhibition of voltage-operated calcium channels [
4,
7,
8,
10]. Similar to the bupivacaine-induced relaxation observed in the current study, bupivacaine inhibits norepinephrine-induced contraction and induces vasorelaxation in isolated vessels precontracted with norepinephrine [
2,
3]. However, as nifedipine or verapamil did not completely abolish bupivacaine-induced relaxation in the current study, further studies are required on other cellular mechanisms of bupivacaine-induced relaxation besides the inhibition of voltage-operated calcium channels. Smooth muscle contraction is regulated by a calciumdependent mechanism associated with [Ca
2+]
i and calcium sensitization of contractile protein [
21]. Therefore, the inhibitory effect of bupivacaine does not appear to be limited to a decrease in [Ca
2+]
i. In the present study using simultaneous [Ca
2+]
i and tension measurement, the magnitude of bupivacaine-induced relaxation was similar to that of the bupivacaine-induced [Ca
2+]
i decrease in KCl-induced precontracted aortas (
Fig. 6B), whereas the magnitude of bupivacaine-induced relaxation was higher than that of the bupivacaine-induced [Ca
2+]
i decrease in phenylephrine-induced precontracted aortas (
Fig. 5B). These results suggest that bupivacaine-induced relaxation may be associated with the inhibition of calcium sensitization involved in phenylephrine-induced contraction. In addition, Rho-kinase and protein kinase C inhibitors attenuate mepivacaine-induced vasoconstriction via the inhibition of calcium sensitization, suggesting that decreased calcium sensitization seems to be associated with vasodilation [
15]. Because phenylephrine-induced contraction involves calcium sensitization associated with a pathway mediated by protein kinase C and Rho kinase, further research is needed regarding whether bupivacaine-induced vasodilation in aortas precontracted with phenylephrine may be associated with the inhibitory effect of bupivacaine on Rhokinase-and protein kinase C-mediated calcium sensitization induced by Rho-kinase activator NaF or protein kinase C activator phorbol 12,13-dibutyrate [
21].
KCl-induced contraction is mainly mediated by calcium influx via voltage-operated calcium channels [
22]. Contraction induced by a receptor-mediated contractile agonist is mediated by calcium influx via receptor-operated calcium channels and calcium release from the sarcoplasmic reticulum [
22]. In agreement with previous findings that verapamil attenuates phenylephrine-induced contraction and that norepinephrine-induced contraction involves calcium influx via both voltage- and receptor-operated calcium channels, verapamil attenuated phenylephrine-induced contraction (
Fig. 4), suggesting that phenylephrine-induced contraction appears to involve partial activation of voltage-operated calcium channels [
12,
23]. In addition, norepinephrine-induced contraction involves the opening of verapamil-sensitive L-type calcium channels [
22]. A supraclinical dose of alfentanil and etomidate attenuates phenylephrine-induced contraction via an inhibitory effect on calcium influx via voltage-operated calcium channels [
12,
13]. Taking into consideration previous reports and the current results, particularly our finding that the magnitude of bupivacaine-induced relaxation was more potent in endothelium-denuded aortas precontracted with 100 mM KCl than with phenylephrine (3 × 10
-6 M) (
Fig. 3), bupivacaine-induced relaxation in the current study appears to be mediated, in part, by the inhibition of voltage-operated calcium channels in endothelium-denuded aortas precontracted with phenylephrine [
12,
22,
23]. Further studies are needed on the signal transduction pathway associated with the inhibition of voltage-operated calcium channels induced by a toxic dose of bupivacaine in vascular smooth muscle.
The clinical relevance of the current
in vitro study should be tempered because of the following factors: 1) we used aortas as conduit vessels in this study, whereas organ blood flow is controlled by small resistance arterioles; 2) we used endothelium-denuded aortas, whereas endothelial nitric oxide release due to blood flow-induced shear stress
in vivo could enhance toxic-dose bupivacaine-induced vasodilation; and 3) differences in species (rats versus humans) and experimental setting (
in vitro versus
in vivo) could modify the current results [
24,
25]. Even with these limitations, the concentration (1.61 × 10
-5 to 9.51 × 10
-4 M) of bupivacaine that produced vasodilation exceeds the plasma concentration of bupivacaine (6.4 × 10
-6 to 1.85 × 10
-5 M) required to produce myocardial depression due to bupivacaine-induced systemic toxicity; therefore, toxicdose bupivacaine-induced vasodilation induced by the inhibition of calcium sensitization on vascular smooth muscle may contribute to cardiovascular collapse due to bupivacaine systemic toxicity [
26].
In conclusion, toxic-dose bupivacaine-induced vasodilation seems to be mediated by the inhibition of calcium sensitization evoked by phenylephrine in isolated endothelium-denuded rat aortas. In addition, potassium channel inhibitors had no effect on bupivacaine-induced relaxation. Toxic-dose bupivacaine-induced relaxation appears to be partially associated with the inhibition of voltage-dependent calcium channel activated by phenylephrine in endothelium-denuded rat aortas.
Go to :











 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download