Abstract
Brachial plexus injury is a potential complication of a brachial plexus block or vessel puncture. It results from direct needle trauma, neurotoxicity of injection agents and hematoma formation. The neurological presentation may range from minor transient pain to severe sensory disturbance or motor loss with poor recovery. The management includes conservative treatment and surgical exploration. Especially if a hematoma forms, it should be removed promptly. Comprehensive knowledge of anatomy and adept skills are crucial to avoid nerve injuries. Whenever possible, the patient should not be heavily sedated and should be encouraged to immediately inform the doctor of any experience of numbness/paresthesia during the nerve block or vessel puncture.
The brachial plexus block (BPB) is a popular technique for providing operative anesthesia and pain control of the upper extremities [1,2,3]. Also subclavian or jugular vein catheterization is widely performed by anesthesiologists [4,5]. However, these procedures are not always safe and may cause various complications including brachial plexus injury (BPI) [6,7,8,9]. Additionally, the axillary arteriography, which has been used if the femoral route is not available, may also cause BPI [10].
Nerve injury is a serious complication. The patient with BPI may suffer only minor transient pain. However, the injury may result in permanent sensory disturbance or motor loss with poor recovery [4,11]. This paper presents literature reviews of BPI as a complication after BPB or vessel puncture including mechanism, clinical course, management and methods for prevention.
A PubMed search was performed from 1950 to 2014 using the search terms brachial plexus, brachial plexus injury, brachial plexus neuropathies, brachial plexus block, nerve block, and different structures relevant to this review including subclavian vein, jugular vein and axillary artery.
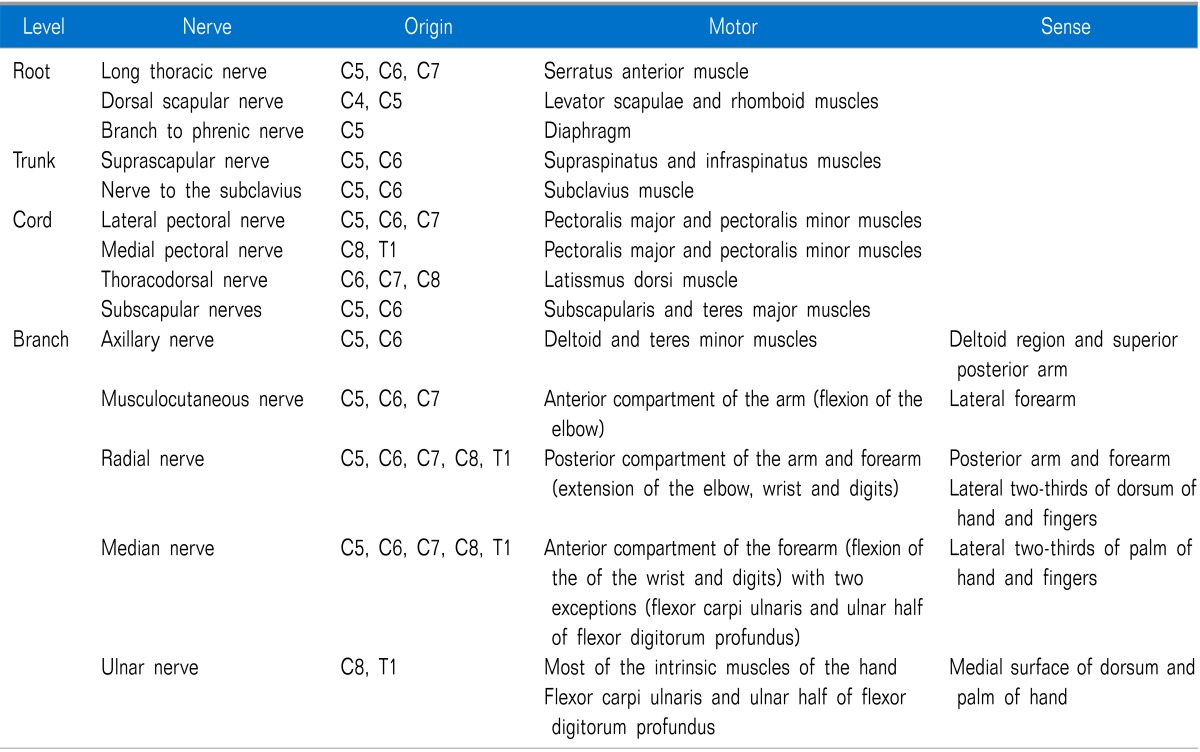
The brachial plexus is formed by the union of the anterior primary divisions (ventral rami) of the C5-C8 and T1 spinal nerves with variable contributions from the C4 and T2 nerves. As the nerve roots leave the intervertebral foramina, they form trunks, divisions, cords, branches and terminal nerves, in that order [12]. It is important to understand how the brachial plexus provides sensory and motor innervations to the upper limbs (Table 1) [13,14].
If the BPI is to happen during BPB or vessel puncture, it could be more common in distal nerves to the intraforaminal dorsal root ganglion. A supraclavicular injury usually occurs at the root and trunk levels, while an infraclavicular injury typically occurs at distal to the cord level.
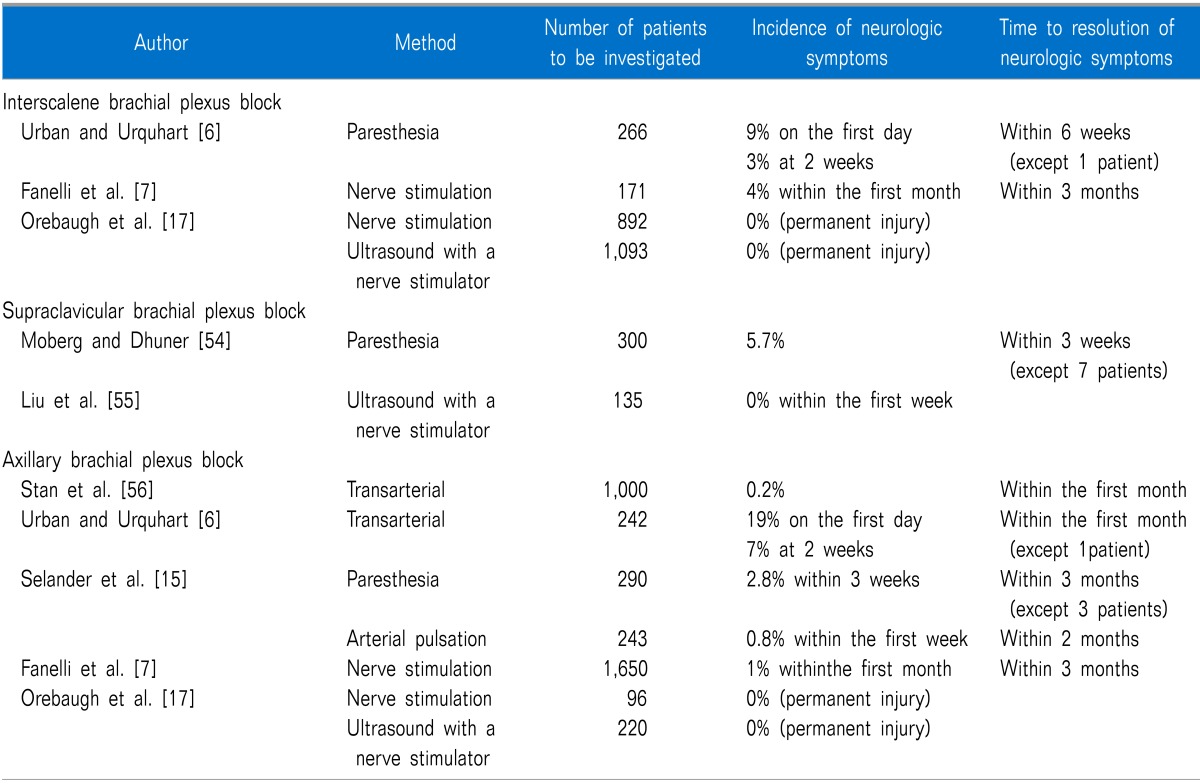
Several studies have evaluated the incidence of postoperative neurological symptoms after BPB for surgery (Table 2). The incidences are quite variable and may be influenced by the used methods to identify neurologic symptoms.
The locus of BPB seems to influence the incidence of nerve injury. Fanelli et al. [7] prospectively studied interscalene blocks (n = 171) and axillary blocks (n = 1,650) using multiple injection technique with a nerve stimulator. The relative incidence of neurologic dysfunction was higher in patients receiving interscalene blocks (4%) than in patients receiving axillary blocks (1%).
Seeking paresthesia during a nerve block may increase the risk of post-anesthetic neurological sequelae in itself. Selander et al. [15] studied the frequency of postanesthetic nerve lesions after axillary BPB with/without searching for paresthesia. They found that all patients with nerve injury had reported painful paresthesia during the blocking procedure. Ultrasonographic guidance may improve the success rate and reduce BPB-related seizures [16,17]. However, it is unclear if it can actually reduce the incidence of neurological sequelae.
Patients on heavy sedation or general anesthesia may be at increased risk of nerve injury. Ben-David et al. [18] investigated adult patients (aged > 14 years) undergoing an axillary block and found that patients with awake or light sedation were less predisposed to a neurological injury than fully anesthetized patients (2.6% vs. 4.1%). Pediatric patients who had a block under general anesthesia had the highest rate of postoperative neurological complications (10.3%).
The location of the subclavian vein between the clavicle and the first rib provides a convenient place for central venous catheterizations. Brachial plexus divisions lie superior to the subclavian artery and vein at the level of the supraclavicular triangle. The complications of subclavian vein catheterization include arterial puncture, pneumothorax, hemothorax, catheter malposition and so on. Of them, the reported incidences of nerve injury have been relatively rare (0-0.6%) [19,20]. But several case reports presented the possibility of BPI as a procedure complication [4,5,8].
Percutaneous catheterizations of the internal jugular vein have been shown to be relatively safe with a lower incidence of serious complications than the subclavian route [9]. However, another report showed similar overall rates of failure and complication between the subclavian and jugular approaches [21]. There are also several case reports on BPI after internal jugular vein catheterization [22,23].
The axillary artery route can be used for an arteriography if the femoral route is contraindicated or not available, such as in those patients with aortoiliac disease, aneurysm in the abdominal aorta or coarcted aorta. Axillary arteriography may also cause BPI. Chitwood et al. [10] reviewed 842 transaxillary arteriography cases and found 14 (1.7%) patients with nerve injuries.
A suspected mechanism during nerve block is a direct needle trauma. Patients typically have acute symptoms at the time of needle placement with painful electric shocks radiating distally down the limb in the distribution of the nerve [3,11]. It is believed that paresthesia is a marker for a potentially traumatic needle contact with a nerve. Therefore, the technique using the elicitation of a paresthesia may be associated with postanesthetic neurological sequelae [15].
A direct needle trauma can also occur as a result after repeated attempts of subclavian vein catheterization [4,5,8,24,25,26,27,28,29]. The subclavian vein is separated from the subclavian artery and brachial plexus by the anterior scalene muscle. However, a misdirection of the needle such as not parallel to the coronal plane, too far laterally or too deeply can cause damage to the nerve [5]. Anatomical variations in the brachial plexus may contribute to the nerve injury too [8].
The nature of the injection agent is another important factor. In an animal study, both extrafascicular injection and extraneural placement of a neurotoxic agent such as ropivacaine caused nerve damage with focal demyelination. Intrafascicular injection of ropivacaine resulted in more severe damage with histological abnormality including edema, axonal destruction and wallerian degeneration. In contrast, normal saline resulted only in an intraneural edema without demyelination or wallerian degeneration even if it was injected into the nerve fascicle [30]. Obviously, intrafascicular injections of neurotoxic agents cause more damage to the nerve than extrafascicular or extraneural injections [30,31].
Experimental studies have shown that all local anesthetics (efocaine, lidocaine, tetracaine, bupivacaine, mepivacaine, ropivacaine, etc) are potentially neurotoxic [32,33,34,35]. In particular, lidocaine and tetracaine seems to have a greater potential for neurotoxicity than other local anesthetics [33,34]. The neurotoxic effects of bupivacaine and ropivacaine may be more reversible compared with lidocaine and mepivacaine [34]. The degree of damage is associated with the concentration of local anesthetics and time of exposure of the nerve to the local anesthetics [34,35].
Supplemental epinephrine causes vasoconstriction and reduces peripheral nerve blood flow. It can result in nerve ischemia and potentiate local anesthetic-induced toxicity. Therefore, it appears reasonable to use lower doses of adjuvant epinephrine in peripheral nerve block [36]. The use of an unsterile or contaminated agent, and oil-based preparations may lead to a nerve injury, too [3].
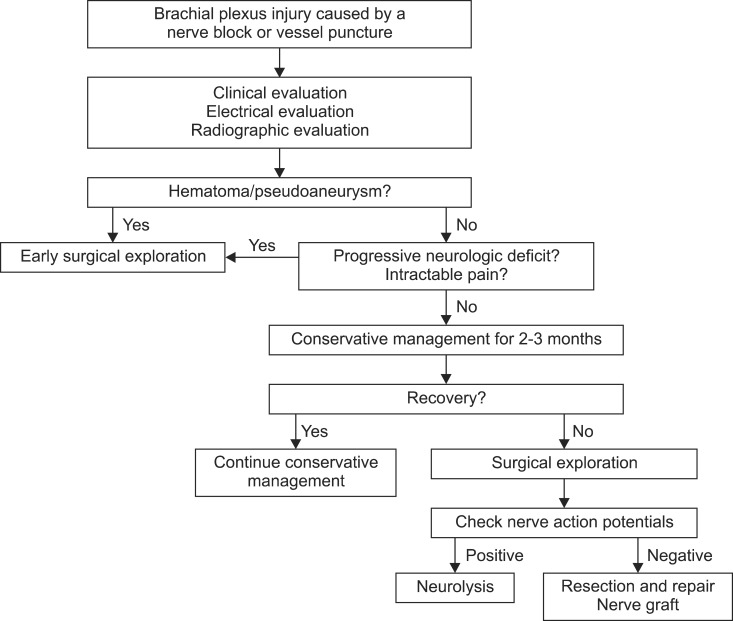
Hematoma or pseudoaneurysm may be formed after BPB, axillary arteriography or subclavian/jugular catheterization. They could pressurize the nerve and induce ischemic changes [4,11,22,37]. The myelin sheath may become damaged even with trivial injuries, thereby leading to a conduction block. Wallerian degeneration may be occurred if the nerve remains compressed for 24 hours. However, axonal death may occur after a much shorter period of compression [37].
Tsao and Wilbourn [11] proposed the medial brachial fascial compartment syndrome as an injury mechanism following axillary regional block or angiography. The medial brachial fascial compartment is formed by tough brachial fascia extending from the axilla to the elbow. A needle injury of the axillary artery results in slow leakage of blood within this compartment. Increased pressure in the compartment can be sufficient to compromise microcirculation without increasing intra-arterial pressure. Thus, distal circulation and pulses may be normal in the presence of considerable but imperceptible nerve damage in most patients. This leads to a delayed presentation onset in patients after the axillary block or angiography.
A direct injection into the nerve may result in immediate severe pain radiating distally down the limb and neuromotor/neurosensory dysfunction in the distribution of the nerve [5,11]. The patient may continue to complain of pain, paresthesia or weakness on the affected side even after the removal of needle or catheter [8]. However, if the hematoma is formed by an injury, symptoms may be delayed and the patient may present only after the hematoma has expanded to a size large enough to compress the nerves. It can occur after hours or days [4,11].
After the nerve injury, the patient can complain of only mild numbness and tingling sensation. But pain is present in the majority of cases and may be described as a shooting or burning sensation. It can be exacerbated by physical factors such as pressure or touch. Severe allodynia or paresthesia can be developed with difficulty of motor activity [4,8,38].
If small fibers are involved, neurosensory loss to pin prick, hyperalgesia, hypesthesia, paresthesia and disturbed sympathetic innervation may be found. On the other hand, if large fibers are involved, motor power disturbance and loss of touch/vibration sense can be more common. In general, chemical toxicity leads to injury in small fibers, while excessive pressure leads to injury in large fibers [3].
Neurological symptoms have been reported according to the procedures in a varied distribution spectrum of the brachial plexus. Tsao and Wilbourn [11] noted that an nerve injury following an axillary regional block primarily involved the median nerve alone or in combination with the ulnar nerve and that no other nerve was injured in isolation. Another study reported that motor weakness after axillary angiography was most commonly involved in the median nerve distribution, followed by the ulnar nerve [37]. This could be explained by the medial brachial fascial compartment. Nerves at the level of the upper arm exit the medial brachial fascial compartment in the sequence (from proximal to distal) of musculocutaneous, axillary and radial. However, the median and ulnar nerves travel within the compartment to the elbow level. Therefore, the median and ulnar nerve are preferentially involved if there is a hematoma within this compartment [11]. On the other hand, the lower trunk, especially the ulnar nerve which arises from the lower trunk fibers, is the most vulnerable to be injured during infraclavicular subclavian vein catheterization [4,5]. But the upper trunk of brachial plexus could be injured during subclavian vein catheterization due to anatomical variations [8].
Connective tissue proliferation and scar formation may occur with time. The early sign of axonal regeneration with the reduplication of Schwann cells and axonal sprouting could be seen 1 to 2 weeks after nerve injury and the further regeneration with an improvement of initial symptoms is usually well advanced by 2 months after the injury. However, the regeneration is often inadequate and the initial loss could persist with severe sensory and/or motor disturbances. In such case, it may not be improved without a surgical intervention [39,40].
The past medical history, social habits, a detailed history associated to the nerve injury, and a review of systems should be evaluated in detail. Also it is important to conduct a physical examination about the sensory and motor disturbances including the level and severity of injury and changes of symptoms [39,41].
Electromyography can be used to diagnose BPI [8,11]. Electromyography may show complete or decreased functional loss if the nerve is stimulated proximal to the lesion, while normal response may be shown if the nerve is stimulated distal to the lesion. Classical signs of a denervation may not be detectable for up to 1-2 weeks after acute nerve transection. Therefore, electromyography should be performed at least 3-4 weeks after nerve damage [37]. Nerve conduction study can be helpful to diagnose nerve injuries, too. It is considered to be abnormal if the responses are less than 50% compared with the contralateral side or age-adjusted normal values [11].
Magnetic resonance imaging and ultrasonography are further methods to diagnose BPI. Magnetic resonance imaging can visualize an edematous nerve or a hematoma formation compressing the nerve [4,11,37]. On ultrasonography, normal brachial plexus appears as a hypoechoic mass containing a tubular structure on a transverse scan and a longitudinal hypoechoic structure containing small hyperechoic linings on a longitudinal scan. The injured nerve can be visualized as an enlarged and edematous nerve with loss of the hyperechoic lining or the discontinuity of the nerve [42].
The BPI must be differentiated from those of the original injury, operation or misuse of a tourniquet. Tourniquet injury usually affects the radial nerve with/without the median and ulnar nerves and is correlated with a good outcome in most cases [11].
In general, the management for BPI is similar to that of other nerve lesions. The treatment of BPI should be individualized for each patient depending on the injury site, degree of damage, lag between injury and repair, age, occupation, and so on.
Conservative management is indicated if the lesions in continuity are non-degenerative or if the fascicles are intact. The treatment with medications consists of both opioid and non-opioid analgesics. Other drugs to control acute neuropathic pain include antidepressants (tricyclic antidepressants, serotonin and norepinephrine reuptake inhibitors), antiepileptic drugs (gabapentin, phenytoin, pregabalin), membrane stabilizers (intravenous lidocaine), ketamine and systemic glucocorticoid [43]. In addition, physiotherapy should be immediately initiated to prevent atonia [4,44,45]. Galvanic stimulation to the affected muscles and nerve ganglion blocks has been reported to provide symptom relief [3,46].
If a hematoma forms, its prompt evacuation may significantly reduce symptoms. Chitwood et al. [10] reported a better prognosis (eight-fold) in patients with medial brachial fascial compartment syndrome when a surgical evacuation was conducted within 4 hours of injury compared with after 4 hours from injury. Sensory changes after axillary artery puncture should be considered as an early indicator of developing BPI due to hematoma formation [37].
Lesions should be treated with surgical intervention if sensory or motor disturbances persist with a severe degeneration. An intraoperative examination of the nerve action potential is a useful method for the assessment of the neural function. The presence of nerve action potential beyond an injury indicates a preserved axonal function with good recovery. On the other hand, the absence of nerve action potential means an inadequate regeneration with poor recovery. Preganglionic injuries are usually treated with nerve transfers, while postganglionic lesions are treated with resection and repair, or nerve graft (an excision of the damaged segment and nerve autograft between two nerve ends) (Fig. 1) [39,41,47,48].
Sufficient knowledge of anatomy, understanding of procedure, and adept skills in needle placement are essential for prevention of BPI. Only medical agents that have been proven reliability and safety should be used for nerve block. New agents should be used as cautiously as possible [3].
Nerve blocks should be performed without searching for paresthesia. The paresthesia method for the nerve block may increase the risk of postanesthetic neurological sequelae compared with no paresthesia methods [15], though paresthesia at the time of needle insertion does not always lead to postanesthesia nerve injury [49]. Currently, the paresthesia technique is not widely used for BPB. Instead of it, nerve stimulation has been the standard method for decades. In recent years, ultrasound guided nerve block has become popular with the improvements of ultrasound technology. Ultrasound allows a direct visualization of various peripheral nerves and localization of the local anesthetics. This may increase the success rate, decrease performance time and reduce the volume of local anesthetics [50,51]. However, further studies are needed to clarify the issue if ultrasound guidance could actually reduce the risk of nerve injury.
The type of needle seems to influence nerve penetration. Hirasawa et al. [52] studied the effect of three different types of needles (a short-bevelled, a long-bevelled and a tapered needle) on the degree of nerve injury in rabbits. A tapered needle did not cause any damage or tearing of the nerve fibers and resulted in the lowest level of damage to the perineurium. With both short- and long-bevelled needles, neural damage was reduced when the face of the bevel was inserted parallel to the nerve fibers.
Discovery of complications of BPB may be missed, since BPB is frequently performed as an out-patient procedure. Patients may confuse symptoms of BPI following BPB with symptoms caused by the original injury or operation. A careful observation and follow-up are imperative after the operation as well as during procedures [3,53].
While performing a subclavian catheterization, the interventionist should move the needle parallel to the coronal plane and avoid entering extreme depths [8]. A subclavian venipuncture should not be too laterally or deeply attempted [4]. If a fist attempt is failed, repeated attempts in the same region should be avoided. Multiple attempts could increase the chance of complications, because an anatomical anomaly may be present [8]. Puncturing the subclavian artery may lead to hematoma formation, which may compress the nerves and cause neurologic damage. If the patient has a prolonged bleeding time, coagulopathy should be corrected first before the performance of subclavian venipuncture or extrathoracic veins like the jugular vein should be used for catheterization [4]. The chances of a BPI are really greater when an inexperienced physician is performing a subclavian vein catheterization [4].
A brachial plexus injury is a potential complication of a nerve block or vessel puncture. With an early recognition of nerve injuries, an appropriate management should be performed in order to reduce neurologic deficits and to maximize recovery. Comprehensive knowledge of anatomy and adept skills are crucial to avoid nerve injuries. Whenever possible, the patient should not be heavily sedated and should be encouraged to immediately inform a practitioner about any experiences of numbness/paresthesia during the nerve block or vessel puncture.
References
1. Iwata T, Mitoro M, Kuzumoto N. Feasibility of early and repeated low-dose interscalene brachial plexus block for residual pain in acute cervical radiculopathy treated with NSAIDS. Korean J Pain. 2014; 27:125–132. PMID: 24748940.

2. Johnson PS, Greifenstein FE. Brachial plexus block anesthesia. J Mich State Med Soc. 1955; 54:1329–1331. PMID: 13271992.
3. Woolley EJ, Vandam LD. Neurological sequelae of brachial plexus nerve block. Ann Surg. 1959; 149:53–60. PMID: 13617908.

4. Karakaya D, Baris S, Güldogus F, Incesu L, Sarihasan B, Tür A. Brachial plexus injury during subclavian vein catheterization for hemodialysis. J Clin Anesth. 2000; 12:220–223. PMID: 10869922.

5. Trentman TL, Rome JD, Messick JM Jr. Brachial plexus neuropathy following attempt at subclavian vein catheterization. Case report. Reg Anesth. 1996; 21:163–165. PMID: 8829409.
6. Urban MK, Urquhart B. Evaluation of brachial plexus anesthesia for upper extremity surgery. Reg Anesth. 1994; 19:175–182. PMID: 7999652.
7. Fanelli G, Casati A, Garancini P, Torri G. Nerve stimulator and multiple injection technique for upper and lower limb blockade: failure rate, patient acceptance, and neurologic complications. Study Group on Regional Anesthesia. Anesth Analg. 1999; 88:847–852. PMID: 10195536.

8. Porzionato A, Montisci M, Manani G. Brachial plexus injury following subclavian vein catheterization: a case report. J Clin Anesth. 2003; 15:582–586. PMID: 14724079.

9. Jernigan WR, Gardner WC, Mahr MM, Milburn JL. Use of the internal jugular vein for placement of central venous catheter. Surg Gynecol Obstet. 1970; 130:520–524. PMID: 5413435.
10. Chitwood RW, Shepard AD, Shetty PC, Burke MW, Reddy DJ, Nypaver TJ, et al. Surgical complications of transaxillary arteriography: a case-control study. J Vasc Surg. 1996; 23:844–849. PMID: 8667506.

11. Tsao BE, Wilbourn AJ. Infraclavicular brachial plexus injury following axillary regional block. Muscle Nerve. 2004; 30:44–48. PMID: 15221877.

12. Madison SJ, Ilfeld BM. Peripheral nerve blocks. In : Butterworth JF, Mackey DC, Wasnick JD, editors. Morgan & Mikhail's clinical anesthesiology. 5th ed. New York (NY): McGraw-Hill;2013. p. 981–983.
13. Jenkins GW, Kemnitz CP, Tortora GJ. Anatomy and physiology from science to life. Hoboken (NJ): John Wiley & Sons, Inc;2007.
14. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014; 27:210–221. PMID: 23959836.

15. Selander D, Edshage S, Wolff T. Paresthesiae or no paresthesiae? Nerve lesions after axillary blocks. Acta Anaesthesiol Scand. 1979; 23:27–33. PMID: 425811.

16. Kapral S, Greher M, Huber G, Willschke H, Kettner S, Kdolsky R, et al. Ultrasonographic guidance improves the success rate of interscalene brachial plexus blockade. Reg Anesth Pain Med. 2008; 33:253–258. PMID: 18433677.

17. Orebaugh SL, Williams BA, Vallejo M, Kentor ML. Adverse outcomes associated with stimulator-based peripheral nerve blocks with versus without ultrasound visualization. Reg Anesth Pain Med. 2009; 34:251–255. PMID: 19587625.

18. Ben-David B, Barak M, Katz Y, Stahl S. A retrospective study of the incidence of neurological injury after axillary brachial plexus block. Pain Pract. 2006; 6:119–123. PMID: 17309720.

19. Bernard RW, Stahl WM. Subclavian vein catheterizations: a prospective study. I. Non-infectious complications. Ann Surg. 1971; 173:184–190. PMID: 5100094.
20. Ryan JA Jr, Abel RM, Abbott WM, Hopkins CC, Chesney TM, Colley R, et al. Catheter complications in total parenteral nutrition. A prospective study of 200 consecutive patients. N Engl J Med. 1974; 290:757–761. PMID: 4205578.

21. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986; 146:259–261. PMID: 3947185.

22. Fuller GN, Dick JP, Colquhoun IR. Brachial plexus compression by hematoma following jugular puncture. Neurology. 1994; 44:775–776. PMID: 8164848.

23. Paschall RM, Mandel S. Brachial plexus injury from percutaneous cannulation of the internal jugular vein. Ann Emerg Med. 1983; 12:58–60. PMID: 6849530.

24. Jackson L, Keats AS. Mechanism of brachial plexus palsy following anesthesia. Anesthesiology. 1965; 26:190–194. PMID: 14261054.

25. García-Fages LC, Castillo J, Gomar C, Villalonga A, Nalda MA. Transient block of the brachial plexus after catheterization of the subclavian vein. Ann Fr Anesth Reanim. 1990; 9:93–94. PMID: 2331089.
26. Govenko FS, Rogulov VA. Injury of the brachial plexus after catheterization of the subclavian vein in a 6-month-old child. Vestn Khir Im I I Grek. 1983; 130:121–122.
27. Defalque RJ. Percutaneous catheterization of the internal jugular vein. Anesth Analg. 1974; 53:116–121. PMID: 4589503.

28. Daily PO, Griepp RB, Shumway NE. Percutaneous internal jugular vein cannulation. Arch Surg. 1970; 101:534–536. PMID: 5457256.

29. Matz R. Complications of determining the central venous pressure. N Engl J Med. 1965; 273:703. PMID: 5827035.

30. Whitlock EL, Brenner MJ, Fox IK, Moradzadeh A, Hunter DA, Mackinnon SE. Ropivacaine-induced peripheral nerve injection injury in the rodent model. Anesth Analg. 2010; 111:214–220. PMID: 20442258.

31. Gentili F, Hudson AR, Hunter D. Clinical and experimental aspects of injection injuries of peripheral nerves. Can J Neurol Sci. 1980; 7:143–151. PMID: 7407720.

32. Nowill WK, Hall H, Stephen CR. Neurological complications following the use of efocaine. AMA Arch Surg. 1953; 67:738–740. PMID: 13103940.

33. Yamashita A, Matsumoto M, Matsumoto S, Itoh M, Kawai K, Sakabe T. A comparison of the neurotoxic effects on the spinal cord of tetracaine, lidocaine, bupivacaine, and ropivacaine administered intrathecally in rabbits. Anesth Analg. 2003; 97:512–519. PMID: 12873946.

34. Radwan IA, Saito S, Goto F. The neurotoxicity of local anesthetics on growing neurons: a comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth Analg. 2002; 94:319–324. PMID: 11812691.

35. Selander D. Neurotoxicity of local anesthetics: animal data. Reg Anesth. 1993; 18:461–468. PMID: 8110648.
36. Neal JM. Effects of epinephrine in local anesthetics on the central and peripheral nervous systems: neurotoxicity and neural blood flow. Reg Anesth Pain Med. 2003; 28:124–134. PMID: 12677623.

37. O'Keefe DM. Brachial plexus injury following axillary arteriography. Case report and review of the literature. J Neurosurg. 1980; 53:853–857. PMID: 7441349.
38. Reiss W, Kurapati S, Shariat A, Hadzic A. Nerve injury complicating ultrasound/electrostimulation-guided supraclavicular brachial plexus block. Reg Anesth Pain Med. 2010; 35:400–401. PMID: 20607905.

39. Spinner RJ, Kline DG. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000; 23:680–695. PMID: 10797390.

40. Gentili F, Hudson AR, Kline D, Hunter D. Early changes following injection injury of peripheral nerves. Can J Surg. 1980; 23:177–182. PMID: 7363181.
41. Yoshikawa T, Hayashi N, Yamamoto S, Tajiri Y, Yoshioka N, Masumoto T, et al. Brachial plexus injury: clinical manifestations, conventional imaging findings, and the latest imaging techniques. Radiographics. 2006; 26(Suppl 1):S133–S143. PMID: 17050511.

42. Shafighi M, Gurunluoglu R, Ninkovic M, Mallouhi A, Bodner G. Ultrasonography for depiction of brachial plexus injury. J Ultrasound Med. 2003; 22:631–634. PMID: 12795559.

43. Gray P. Acute neuropathic pain: diagnosis and treatment. Curr Opin Anaesthesiol. 2008; 21:590–595. PMID: 18784484.

44. Dhir S, Tureanu L, Stewart SA. Axillary brachial plexus block complicated by cervical disc protrusion and radial nerve injury. Acta Anaesthesiol Scand. 2009; 53:411. PMID: 19243339.

45. Kim DH, Murovic JA, Kline DG. Brachial plexus injury: mechanisms, surgical treatment and outcomes. J Korean Neurosurg Soc. 2004; 36:177–185.
46. Po BT, Hansen HR. Iatrogenic brachial plexus injury: a survey of the literature and of pertinent cases. Anesth Analg. 1969; 48:915–922. PMID: 5391386.
47. Hems T. Nerve transfers for traumatic brachial plexus injury: advantages and problems. J Hand Microsurg. 2011; 3:6–10. PMID: 22654410.

48. Wilkinson MC, Birch R, Bonney G. Brachial plexus injury: when to amputate? Injury. 1993; 24:603–605. PMID: 8288380.

49. Pearce H, Lindsay D, Leslie K. Axillary brachial plexus block in two hundred consecutive patients. Anaesth Intensive Care. 1996; 24:453–458. PMID: 8862642.

50. Gelfand HJ, Ouanes JP, Lesley MR, Ko PS, Murphy JD, Sumida SM, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth. 2011; 23:90–96. PMID: 21377070.

51. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009; 22:655–660. PMID: 19550303.

52. Hirasawa Y, Katsumi Y, Küsswetter W, Sprotte G. Experimental studies on peripheral nerve injuries caused by injection needles. Reg Anaesth. 1990; 13:11–15. PMID: 2305114.
53. Dooley J, Fingerman M, Melton S, Klein SM. Contralateral local anesthetic spread from an outpatient interscalene catheter. Can J Anaesth. 2010; 57:936–939. PMID: 20652841.

54. Moberg E, Dhuner KG. Brachial plexus block analgesia with xylocaine. J Bone Joint Surg Am. 1951; 33-A:884–888. PMID: 14880542.

55. Liu SS, YaDeau JT, Shaw PM, Wilfred S, Shetty T, Gordon M. Incidence of unintentional intraneural injection and postoperative neurological complications with ultrasound-guided interscalene and supraclavicular nerve blocks. Anaesthesia. 2011; 66:168–174. PMID: 21320084.

56. Stan TC, Krantz MA, Solomon DL, Poulos JG, Chaouki K. The incidence of neurovascular complications following axillary brachial plexus block using a transarterial approach. A prospective study of 1,000 consecutive patients. Reg Anesth. 1995; 20:486–492. PMID: 8608066.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download