INTRODUCTION
FACTORS AFFECTING SPINAL OPIOID BIOAVAILABILITY
CLINICAL PHARMACOLOGY OF SPINAL OPIOIDS ALONE AND WITH LOCAL ANESTHETICS
EVIDENCE-BASED RECOMMENDATIONS FOR CORRECT OPIOID SELECTION
1. PROSPECT GROUP (Procedure-Specific Postoperative Pain Management: http://www.postoppain.org)
Procedure-specific recommendations that take into consideration differences in the characteristics, location and severity of pain associated with different surgical procedures.
Evidence from a systematic review is supplemented with transferable evidence and expert knowledge from a Working Group of surgeons and anesthesiologists.
The Prospect Working Group formulates consensus recommendations using established methods for group decision-making (Delphi method, Nominal Group Process).
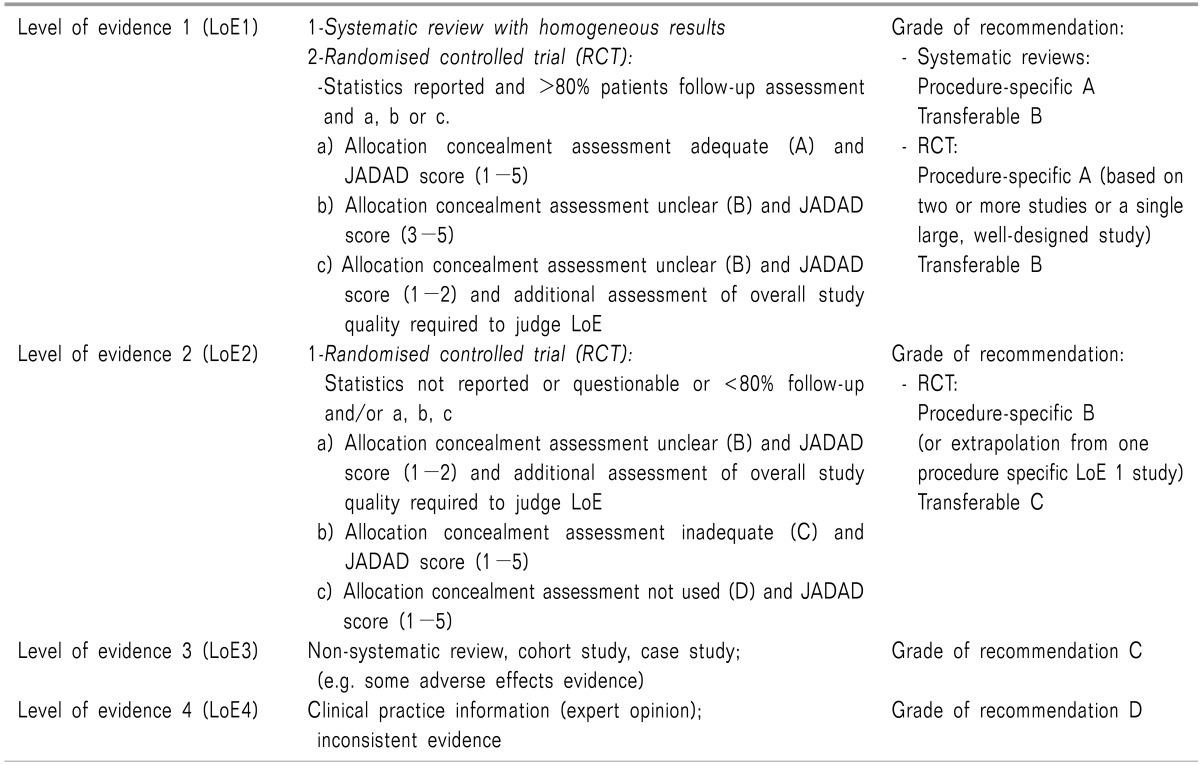
Recommendations are graded to indicate their strength (A-D).
Recommendations are provided with an explanation of the evidence on which they are based, including the level (LoE 1-4) and source of evidence (procedure-specific or transferable).
All evidence from systematic reviews, as well as transferable evidence, is summarized and abstracts of all references are provided.
Studies included in the reviews are assessed and assigned a level of evidence, with the study design, quality, consistency and directness taken into consideration.
Procedure-specific evidence, transferable evidence and clinical practice information (expert opinion) are clearly separated.
Benefits and damages of different interventions are indicated with a system of ticks and crosses, and the balance of benefits and damages is considered when formulating the recommendations.
Evidence and recommendations are freely accessible on the Internet at www.postoppain.org.
1) Recommendations for post-thoracotomy pain
Paravertebral blockade with LA and pre- and intraoperative thoracic epidural LA plus a strong opioid are recommended based on a reduction in pain compared with postoperative administration alone (Grade A).
Thoracic epidural LA plus a strong opioid is recommended as a pre-operative bolus followed by an infusion continued for 2-3 days postoperatively based on a reduction in pain compared with systemic analgesia (Grade A).
Thoracic epidural LA plus opioid is recommended in preference to a spinal strong opioid based on evidence that the analgesic effect of thoracic epidural analgesia has a longer duration than 24 h (Grade A).
A pre-operative single bolus of a spinal strong opioid is recommended as part of a multi-analgesic regimen (Grade A) when epidural analgesia or paravertebral block are not possible for any reason (Grade D). Repeated perioperative doses by the spinal route are not recommended because they are not considered to be safe or practical (Grade D).
Spinal opioids are recommended in preference to intravenous PCA analgesia based on a greater decrease in pain for up to 24 hours, with no difference in respiratory function (Grade A).
A lumbar epidural strong opioid is not recommended as the first choice based on evidence that the thoracic epidural route is more effective for pain relief (Grade A). However, there is procedure-specific evidence that a lumbar hydrophilic strong opioid reduces pain better compared to systemic analgesia.
Epidural epinephrine is recommended if a low dose of epidural LA and/or an opioid is used (Grade B).
2. An updated report by the American Society of Anesthesiologist Task Force on Acute Pain Management
Table 2
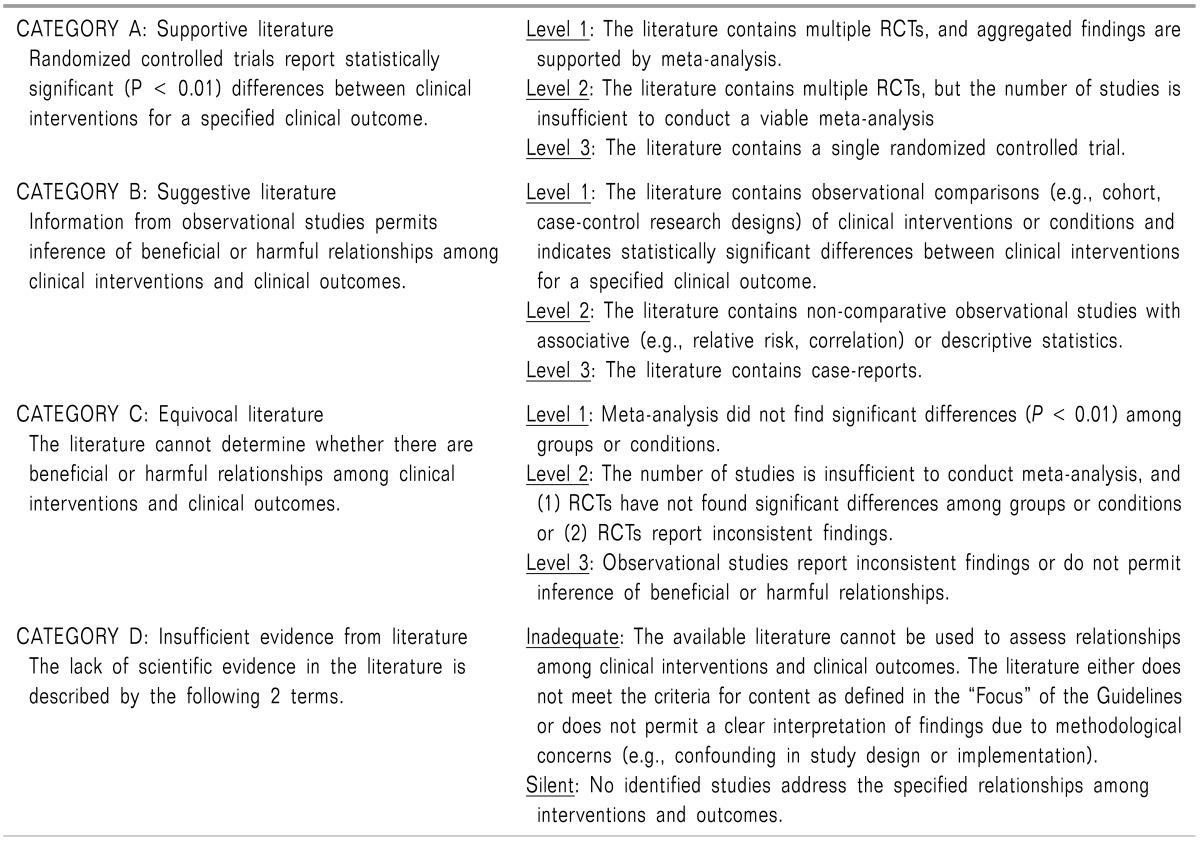
Meta-analyses of RTCs report either improved pain relief or an increased frequency of pruritus in comparisons of post-incision epidural morphine vs. a saline placebo (Category A1 evidence). Findings for the frequency of vomiting and nausea were equivocal (Category C1 evidence).
Meta-analyses of RTCs comparing post-incision IM morphine with epidural morphine report improved pain relief and a higher frequency of pruritus for the epidural route (Category A1 evidence).
RCTs report improved pain relief when pre-incision epidural or intrathecal morphine is used compared with pre-incision oral, intravenous, or intramuscular (IM) morphine (Category A2 evidence).
Meta-analyses of RTCs report improved pain scores and a higher frequency of either pruritus or urinary retention when postoperative epidural morphine is compared with IM morphine (Category A3 evidence). Findings with regard to the frequency of vomiting and nausea were equivocal (Category C2 evidence).
3. Practice guidelines for the management of respiratory depression associated with neuraxial opioid administration
1) Prevention of respiratory depression after neuraxial opioid administration [39]
- Careful attention is required with regard to a history of sleep apnea, coexisting diseases or conditions such as obesity or diabetes mellitus, as well as current medications, especially preoperative opioids, and adverse effects after previous opioid administration.
- Patients treated with non-invasive positive airway pressure for sleep apnea should bring their own equipment to the hospital.
- Single-shot neuraxial opioids could be safely used in place of parenteral opioids without increasing the risk of hypoxemia or respiratory depression.
- Single-shot neuraxial sufentanil or fentanyl could be safe alternatives to single-shot neuraxial morphine.
- If available, extended-release epidural morphine could be used in place of IV or immediate-release epidural morphine; however, extended monitoring is needed.
- Epidural opioids as a continuous infusion are a better choice than parenteral opioids for anesthesia and analgesia in terms of decreasing the risk of respiratory depression.
- If available, adequate doses of a continuous epidural infusion of fentanyl or sufentanil can be used in place of a continuous infusion of hydromorphone or morphine without increasing the risk of respiratory depression.
- Epidural or intrathecal hydromorphone or morphine should not be given to outpatient surgical patients.
- The first decision to minimize the risk of respiratory depression is to select the lowest efficacious dose of neuraxial opioids.
- Other drugs such as hypnotics and parenteral opioids should be administered with caution in the presence of neuraxial opioids.
-The concomitant administration of neuraxial opioids and either parenteral opioids, sedatives or magnesium requires an increased level of monitoring in terms of the duration, intensity or the use of additional methods.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download