Abstract
Epidural adhesions cause pain by interfering with the free movement of the spinal nerves and increasing neural sensitivity as a consequence of neural compression. To remove adhesions and deliver injected drugs to target sites, percutaneous epidural adhesiolysis (PEA) is performed in patients who are unresponsive to conservative treatments. We describe four patients who were treated with a newly developed inflatable balloon catheter for more effective PEA and relief of stenosis. In the present patients, treatments with repetitive epidural steroid injection and/or PEA with the Racz catheter or the NaviCath did not yield long-lasting effects or functional improvements. However, PEA and decompression with the inflatable balloon catheter led to maintenance of pain relief for more than seven months and improvements in the functional status with increases in the walking distance. The present case series suggests that the inflatable balloon catheter may be an effective alternative to performing PEA when conventional methods fail to remove adhesions or sufficiently relieve stenosis.
Various etiologies and pathophysiologies contribute to the development of chronic lower back pain and dictate the appropriate treatment. Epidural adhesions are considered one of the most important factors in the pathophysiologies of back pain. Epidural adhesions most commonly arise from postoperative epidural scarring and can also be seen in patients with spinal stenosis and disc herniation [1,2,3]. Epidural adhesions cause pain by interfering with the free movement of the spinal nerves and increasing neural sensitivity as a consequence of neural compression [4]. Although an epidural injection with a local anesthetic agent and glucocorticoid is an effective treatment for chronic lower back pain and/or radicular pain, this therapy often works for only a few weeks or may not improve the patient's functional status [5,6]. It may be attributed to the reason that the injectate cannot spread out to the lesion properly due to the epidural adhesions [7]. Therefore, physicians may performe percutaneous epidural adhesiolysis (PEA) on patients who are unresponsive to existing and temporary treatments, with the goal of eliminating adhesions and allowing the delivery of injections to target sites.
PEA is commonly performed with a Racz catheter or a more steerable navigation catheter (NaviCath®, Myelotec, Inc., Roswell, GA, USA) and has proven to be effective [8,9]. However, the approach and correct placement of these catheters can be difficult in patients with severe adhesions or stenosis, leading to incomplete removal of the adhesions [10]. Moreover, the long-term effects (i.e., over more than 6 months) of this treatment are uncertain and controversial [11]. Importantly, there has not yet been any treatment developed to relieve stenosis itself through a nonsurgical method.
Previously, we have reported that transforaminal balloon treatment results in significant pain relief and functional improvement in patients with chronic refractory lumbar foraminal stenosis [12]. On the basis of this concept, a novel balloon catheter for more effective PEA and decompression was developed: the Zigzag-motion Inflatable Neuroplasty (ZiNeu®, JUVENUI, Seoul, Korea) catheter, which can be adjusted side-to-side and has an inflatable balloon attached to the end of the catheter tip (Fig. 1). In the present report, we describe four patients who suffered from persistent lower back pain radiating to the leg despite repeated conventional epidural steroid injections and other PEA modalities, and who were successfully treated with the inflatable balloon neuroplasty catheter.
A 75-year-old man presented to our clinic with pain in his back, both thighs, and calves that had persisted for seven months. He had a medical history of well-controlled hypertension and a depressive disorder. When he walked for 10 minutes, his pain was aggravated and accompanied by dysesthesia of the feet, and these symptoms were relieved by bending over. His pain score was 8 on the 11-point Numeric Rating Scale (NRS; 0 = no pain, 10 = worst pain imaginable), and his Oswestry Disability Index (ODI; ranging from 0-100; 0 = no disability) score was 56. No abnormal signs were seen on physical examination. Magnetic resonance imaging (MRI) of his lumbar spine revealed central stenosis at the L4-5 level due to a bulging disc, facet arthrosis, and thickening of the ligamentum flavum (Fig. 2). For two years, he had been treated with oral medication, a fentanyl patch, and five sets of epidural steroid injections. In addition, PEA had been performed three times (once with the NaviCath and twice with the Racz catheter). However, the therapeutic effect of these procedures did not last for more than three weeks, and the duration of pain relief was further shortened after a series of procedures. His functional status was worsening, and his ODI score increased to 72.
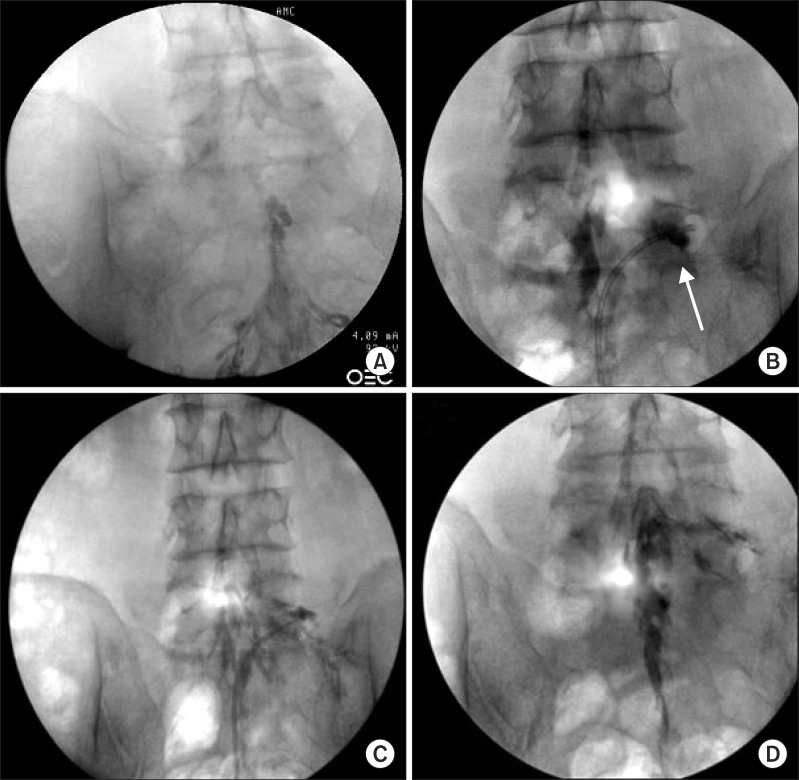
After obtaining the patient's written informed consent, we performed PEA and decompression with the inflatable balloon neuroplasty catheter to reduce his pain and extend the duration of pain relief. The patient was placed in the prone position with a pillow under his abdomen to minimize lumbar lordosis. After sterile preparation for the procedure, a 10 G guide needle, which was specially designed to prevent cutting and skiving of the catheter, was inserted into the epidural space through the sacral hiatus. The epidural space was identified by injection of contrast medium (Omnipaque, Nycomed Imaging AS, Oslo, Norway) under fluoroscopy. A caudal epidurogram, checked before planning the PEA, showed a filling defect from the central epidural space at the L5-S1 level to both L5 intervertebral foramina (Fig. 3A). We performed mechanical adhesiolysis and decompression of the intervertebral foramina with the inflatable balloon neuroplasty catheter in order of precedence (i.e., central anterior epidural space, lateral recess area, and each intervertebral foramen). PEA and decompression were conducted by gentle side-to-side movement of the catheter with ballooning. The balloon was filled with 0.13 ml of contrast agent, and each ballooning process was limited to 5 seconds (Fig. 3B) [12]. The extent of balloon inflation was adjusted by the degree of pain; if moderate to severe pain was noted during balloon inflation, no further attempt at treatment was made for safety reasons. The catheter moved only in the deflated state. After PEA and decompression, the contrast agent in the anterior epidural space spread upward above the level of L5-S1, suggesting that successful adhesiolysis had been achieved (Fig. 3C and 3D). Before removal of the catheter, 6 ml of a mixture of 1% preservative-free lidocaine, 20 mg triamcinolone, and 1,500 IU hyaluronidase was administered via the catheter. There were no complications during the procedure such as bleeding or damage to the dura. On follow-up monitoring after one month, the patient's pain had been reduced from an NRS of 8 to 4. The patient could walk without pain for more than 20 minutes, and his ODI score had decreased to 36. The effect has been sustained for more than 14 months, and the patient is currently being monitored on follow-up.
A 37-year-old man presented to our office with right buttock pain radiating to the leg. There was no weakness or sensory changes, and his lumbar MRI revealed a huge central disc extrusion and sequestration at central L4-5 region (Fig. 4). The patient was first treated with five epidural steroid injections (two by an interlaminar approach and the others by a transforaminal approach). Although this improved his pain level, the effect did not last for more than three weeks; the patient continued to complain of pain with a score of 7 on the NRS, and he could not walk for more than three minutes. His functional score on the ODI was 38.
PEA and decompression with the inflatable balloon neuroplasty catheter were planned and prepared as described above. A caudal epidurogram, performed before the insertion of the inflatable balloon neuroplasty catheter, showed a filling defect in the anterior epidural space above the level of L5. When the inflatable balloon neuroplasty catheter was inserted and advanced to the level of L5, some resistance against the catheter was felt. Gentle adhesiolysis of the anterior epidural space by intermittent balloon inflation was performed as described for Case 1, and the spread of contrast medium above the level of disc herniation was confirmed. At the end of the procedure, a Perifix epidural catheter (B. Braun Melsungen AG, Melsungen, Germany) was left at the target site through the balloon catheter lumen. After test injection of 1 ml lidocaine, 6 ml of a mixture of 1% lidocaine and 1,500 IU hyaluronidase was administered through the Perifix catheter. After 10 to 15 minutes of monitoring, another 4 ml of a mixture of 10% hypertonic saline and 20 mg triamcinolone was injected through the catheter. The catheter was removed on the day of the procedure. There were no complications during the procedure. At the follow-up visit after one month, the patient's pain was found to be reduced and was scored as a 1 on the NRS. The patient had no walking limitations, and his functional status was improved, with a change in the ODI score from 38 to 2. The effect has been maintained for more than 13 months, and the patient is currently being monitored on follow-up.
A 70-year-old man presented with pain in the lower back and both legs. He had undergone a decompressive surgery at the L4-5 level seven years previously. However, his pain had again developed six months prior to presentation. He complained of numbness and a cramping sensation in both legs and could not walk more than 100 meters because of his pain. He scored his pain as 8 on the NRS, and his functional score on the ODI was 40. Left neural foraminal stenosis at the L4-5 and L5-S1 levels and right neural foraminal stenosis at the L3-4 level with degenerative spondylolisthesis, a bulging disc, and facet osteoarthritis were noted by lumbar MRI. The patient had been treated with an epidural steroid injection before visiting our clinic. However, the effect lasted for only two weeks, and his pain was reduced by only 10%.
To relieve the patient's pain and extend the duration of pain relief, PEA and decompression with the inflatable balloon neuroplasty catheter were performed as described for Cases 1 and 2. A caudal epidurogram showed a filling defect from the central epidural space above the L5-S1 level to both the L5 and S1 intervertebral foramina. After anterior epidural adhesiolysis and decompression of both neural foramina by mechanical adhesiolysis and balloon inflation as described in Case 1, contrast agent spread throughout both the L5 and S1 intervertebral foramina and the anterior epidural space above the L4-5 level. At the end of the procedure, 6 ml of a mixture of 1% lidocaine, 20 mg triamcinolone, and 1,500 IU hyaluronidase was administered through the catheter. After two months, the patient's pain scores on the NRS and ODI decreased to 4 and 36, respectively. His walking distance also increased to 500 meters. Interestingly, his pain nearly disappeared three months after the procedure, and he has been doing well without pain for more than 18 months following PEA and decompression with the inflatable balloon neuroplasty catheter.
A 44-year-old female presented with pain in the lower back radiating to the right leg. Her symptoms first developed three years ago. When she walked for 10 minutes, her pain was aggravated and accompanied by numbness with a cramping sensation in the right leg. There was no weakness or sensory changes. No abnormal signs were seen on physical examination. For three years, she had been treated with oral medication and three sets of epidural steroid injections. The effect of the third epidural steroid injection did not last for more than one month. Moreover, PEA with the Racz catheter did not effectively reduce her pain level. The patient continued to complain of pain with a score of 8 on the NRS and she could not walk for more than ten minutes. His functional score on the ODI was 64. Her lumbar MRI revealed central disc extrusion at the L4-5 level with central canal stenosis, and bilateral L5 nerve root compression.
We planned PEA and decompression with the inflatable balloon neuroplasty catheter, and prepared as described above. A caudal epidurogram showed a filling defect from the central epidural space at the L4-5 level to both L5 intervertebral foramina (especially the preganglionic area). Gentle adhesiolysis of the preganglionic area at the right L5 level by intermittent balloon inflation was performed as described above, and the preganglionic spread of contrast medium was confirmed. At the end of the procedure, a Perifix epidural catheter was left at the target site through the balloon catheter lumen. After test injection of 1 ml of lidocaine, 6 ml of a mixture of 1% lidocaine and 1,500 IU hyaluronidase was administered through the Perifix catheter. After 10 to 15 minutes of monitoring, another 4 ml of a mixture of 10% hypertonic saline and 20 mg triamcinolone was injected through the catheter. The Perifix catheter was left in place for a 2-day drug injection. The catheter was then removed on the second day of the procedure, after injection of the same drugs, including the hypertonic solution. There were no complications during the procedure. At the follow-up visit after two months, the patient's pain was found to be reduced and was scored as a 2 on the NRS. The patient reported an improvement in her walking distance, and her functional status was also improved, with a change in the ODI score from 64 to 26. The effect has been maintained for more than seven months, and the patient is currently being monitored on follow-up.
Epidural adhesions or fibrosis commonly develop after surgery, as well as in patients with spinal stenosis or disc herniation [1,2,3]. Several animal models of post-lumbar laminectomy syndrome have shown epidural and perineural scarring and nerve root adherence to the underlying disc and pedicle [13,14]. Furthermore, degenerative changes in the discs, facet joints, and ligamentum flavum cause a disruption of the epidural venous plexus in patients with spinal stenosis [1]. These derangements in the epidural veins lead to recurrent and clinically insignificant microbleeds, which are believed to be the origin of epidural fibrosis [1,2]. In addition, leakage of disc materials into the epidural space following an annular tear leads to acute inflammation, consequent fibrocyte deposition, and epidural adhesions in patients with disc herniation [3]. An epidural adhesion itself is not painful, but it causes pain by trapping spinal nerve roots so that they are placed under tension by movement. In addition, compression of the nerve roots and/or adjacent veins leads to perineural edema and nutritional deficiency. As it progresses, aggravation of neuritis, demyelination, conduction disability, and ectopic neural transmission can occur, eventually leading to the development of neuropathic pain [4].
Hence, PEA has been designed to treat lower back and leg pain originating from an adhesion of the epidural space by removing this adhesion [8]. PEA is performed widely with a shearing-resistant catheter, the Racz catheter [15]. Evidence suggests that PEA is effective in treating refractory lower back pain due to post-lumbar surgery syndrome or spinal stenosis [16]. Recently published experimental biomechanical evaluations of PEA have reported that when performed with the Racz-type catheter, this procedure may be suitable for the targeted application of highly concentrated epidural medications and may have a lavage effect in reducing local inflammatory substances, but it does not exert a true mechanical lysis of adhesions [10]. In order to control the direction of the catheter during the procedure, other instruments have been developed. Among them, the NaviCath, which is more steerable and can be bent to only one side, has been reported to be clinically effective in the treatment of chronic lower back pain that is not responsive to transforaminal epidural injection [9]. However, this procedure has some limitations in patients with a severe degree of adhesion or severe spinal stenosis because of the difficulty in placing the catheter at the target area. In addition, the NaviCath-type catheter cannot be used for additional administration of drugs during 2- or 3-day procedures. A systematic review of spinal endoscopic adhesiolysis has suggested that this procedure is effective in treating post-lumbar surgery syndrome patients who have failed other modalities, including PEA [17]. Nevertheless, epiduroscopic adhesiolysis has not been widely accepted as a treatment due to safety concerns and its high cost [18]. Furthermore, as there are technical issues related to visualization equipment, focal length, and the difficulty in identifying structures, spinal endoscopic adhesiolysis has limited applicability [17].
As shown in Fig. 1, the inflatable balloon neuroplasty catheter can be steered bi-directionally. This feature enables physicians to more easily place the catheter at the target site. In addition, there is an inflatable balloon at the tip of the catheter to remove adhesions or relieve stenosis. Ballooning with a contrast agent helps in visually verifying the degree of adhesion or stenosis by the extent of distortion of the balloon. PEA by ballooning was first described by Song and Lim [19]. These authors performed PEA by ballooning with the Fogarty catheter inserted via R-K needle, which is designed for advancing the Racz catheter to the target lesion, at the sacral hiatus. A randomized double-blind active control trial [12] and a case report [20] have shown that transforaminal decompression using the Fogarty balloon catheter is clinically effective in relieving pain and improving function in patients with spinal stenosis. Previous three-dimensional reconstructed images of the epidural space, confirmed by contrast agent, have shown that the marginal space of a stenotic intervertebral foramen is increased by approximately 98% after transforaminal balloon adhesiolysis in patients with lumbar foraminal stenosis [12]. The Fogarty catheter, however, is not steerable and cannot be used for adhesiolysis of central canal and multilevel stenosis. Moreover, there is no channel for drug injection. The present novel inflatable balloon neuroplasty catheter has been developed to overcome these problems, for more effective adhesiolysis with balloon. As described in Cases 2 and 4 of our present report, the thin epidural catheter can be left at the target site to enable drug injections over several days. Hence, 2- or 3-day regimens with hypertonic saline can be performed. Accordingly, the inflatable balloon neuroplasty catheter can more effectively remove or relieve severe degrees of adhesion. Furthermore, to our knowledge, PEA and decompression using the present inflatable balloon catheter is the only non-surgical method to relieve stenosis itself by increasing the marginal space of the stenotic foramen. Interestingly, the internal dimensions of the ZiNeu catheter are sufficient to allow insertion of the epiduroscope. If the epiduroscope is combined with the ZiNeu catheter, more exact diagnosis and treatment may be performed.
In our present patient subjects, treatments with repeated epidural steroid injections and/or PEA with the Racz catheter or the NaviCath did not yield long-lasting effects or functional improvements. However, as shown in the present case series, PEA and decompression with the inflatable balloon neuroplasty catheter led to maintenance of pain relief for more than seven months and improvements in the patients' functional status, as well as increases in the walking distance. The principal mechanism that alleviated symptoms involved the elimination of adhesions, leading to the restoration of freely moving roots, a reduction of venous stasis, and decreases in perineural edema. The present inflatable balloon neuroplasty catheter can be manipulated by physicians not only vertically but also from side to side. This allows physicians to more effectively perform mechanical adhesiolysis and also to more easily place the catheter at the target lesion, including at the lateral recess, intervertebral foramen, and possibly the extraforaminal space. Furthermore, perineural adhesiolysis, central epidural adhesiolysis, and foraminal decompression are possible by inflating the balloon at the end of the catheter. The present novel balloon catheter has been applied even in multilevel stenosis. As a result, high concentrations of injectable drugs can be more directly delivered to the target lesions. Taken together, therefore, these mechanisms may contribute to the long-lasting effect of pain relief and functional improvements in the present patients.
In conclusion, our current report is the first case series describing the use of the inflatable balloon neuroplasty catheter for PEA and decompression. Our report demonstrates efficacy in all four patient subjects. Although we have provided only anecdotal evidence, our four cases suggest that the inflatable balloon neuroplasty catheter may be an effective alternative for performing PEA when other conventional methods fail to remove adhesions or sufficiently relieve stenosis. Randomized controlled trials with larger sample sizes to assess the effects of this treatment modality on pain improvement, the patient's functional score, and claudication distance are currently underway.
References
1. Berthelot JM, LeGoff B, Maugars Y. The role for radicular veins in nerve root pain is underestimated: limitations of imaging studies. Joint Bone Spine. 2011; 78:115–117. PMID: 21273107.

2. Cooper RG, Freemont AJ, Hoyland JA, Jenkins JP, West CG, Illingworth KJ, et al. Herniated intervertebral disc-associated periradicular fibrosis and vascular abnormalities occur without inflammatory cell infiltration. Spine (Phila Pa 1976). 1995; 20:591–598. PMID: 7604329.

3. Kobayashi S, Baba H, Uchida K, Kokubo Y, Kubota C, Yamada S, et al. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine (Phila Pa 1976). 2005; 30:1699–1705. PMID: 16094269.

4. Anderson SR, Racz GB, Heavner J. Evolution of epidural lysis of adhesions. Pain Physician. 2000; 3:262–270. PMID: 16906184.

5. Armon C, Argoff CE, Samuels J, Backonja MM. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: use of epidural steroid injections to treat radicular lumbosacral pain: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007; 68:723–729. PMID: 17339579.

6. Hong JH, Lee YC, Lee HM, Kang CH. An analysis of the outcome of transforaminal epidural steroid injections in patients with spinal stenosis or herniated intervertebral discs. Korean J Pain. 2008; 21:38–43.

7. Manchikanti L, Bakhit CE. Percutaneous lysis of epidural adhesions. Pain Physician. 2000; 3:46–64. PMID: 16906207.

8. Manchikanti L, Singh V, Bakhit CE, Fellows B. Interventional techniques in the management of chronic pain: part 1.0. Pain Physician. 2000; 3:7–42. PMID: 16906205.
9. Lee JH, Lee SH. Clinical effectiveness of percutaneous adhesiolysis using Navicath for the management of chronic pain due to lumbosacral disc herniation. Pain Physician. 2012; 15:213–221. PMID: 22622905.
10. Birkenmaier C, Baumert S, Schroeder C, Jansson V, Wegener B. A biomechanical evaluation of the epidural neurolysis procedure. Pain Physician. 2012; 15:E89–E97. PMID: 22270752.
11. Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013; 16:S49–S283. PMID: 23615883.
12. Kim SH, Choi WJ, Suh JH, Jeon SR, Hwang CJ, Koh WU, et al. Effects of transforaminal balloon treatment in patients with lumbar foraminal stenosis: a randomized, controlled, double-blind trial. Pain Physician. 2013; 16:213–224. PMID: 23703408.
13. Massie JB, Huang B, Malkmus S, Yaksh TL, Kim CW, Garfin SR, et al. A preclinical post laminectomy rat model mimics the human post laminectomy syndrome. J Neurosci Methods. 2004; 137:283–289. PMID: 15262072.

14. Haq I, Cruz-Almeida Y, Siqueira EB, Norenberg M, Green BA, Levi AD. Postoperative fibrosis after surgical treatment of the porcine spinal cord: a comparison of dural substitutes. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2005; 2:50–54. PMID: 15658126.

15. Heavner JE, Racz GB, Raj P. Percutaneous epidural neuroplasty: prospective evaluation of 0.9% NaCl versus 10% NaCl with or without hyaluronidase. Reg Anesth Pain Med. 1999; 24:202–207. PMID: 10338168.

16. Helm Ii S, Benyamin RM, Chopra P, Deer TR, Justiz R. Percutaneous adhesiolysis in the management of chronic low back pain in post lumbar surgery syndrome and spinal stenosis: a systematic review. Pain Physician. 2012; 15:E435–E462. PMID: 22828693.
17. Helm S, Hayek SM, Colson J, Chopra P, Deer TR, Justiz R, et al. Spinal endoscopic adhesiolysis in post lumbar surgery syndrome: an update of assessment of the evidence. Pain Physician. 2013; 16:SE125–SE150. PMID: 23615889.
18. Kim JD, Jang JH, Jung GH, Kim JY, Jang SJ. Epiduroscopic laser disc and neural decompression. J Neurosurg Rev [serial on the Internet]. 2012. 3. 2012 Mar 22. Available at http://neurosurgicalreview.com/2012/03/epiduroscopic/.
19. Song SO, Lim HJ. Clinical experience of epidural adhesiolysis in patients with failed back surgery syndrome. Korean J Anesthesiol. 2004; 47:547–552.

20. Kim SH, Koh WU, Park SJ, Choi WJ, Suh JH, Leem JG, et al. Clinical experiences of transforaminal balloon decompression for patients with spinal stenosis. Korean J Pain. 2012; 25:55–59. PMID: 22259719.

Fig. 1
The inflatable balloon neuroplasty (ZiNeu) catheter. This instrument can be adjusted from side to side and has an inflatable balloon (arrow) attached to the end of the catheter tip. It also has a channel (arrow-head) to inject drugs or to leave another catheter at the target site for two- or three-day regimens.
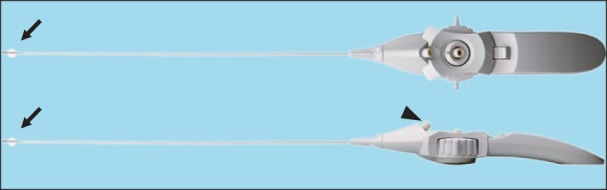
Fig. 2
T2-weighted magnetic resonance images (MRI) of the lumbar spine in a 75-year-old man suffering from pain in his back, both thighs, and calves that persisted for seven months. (A) Sagittal and (B) axial views of the MRI show central stenosis at the L4-5 level caused by a bulging disc, facet arthrosis, and thickening of the ligamentum flavum.
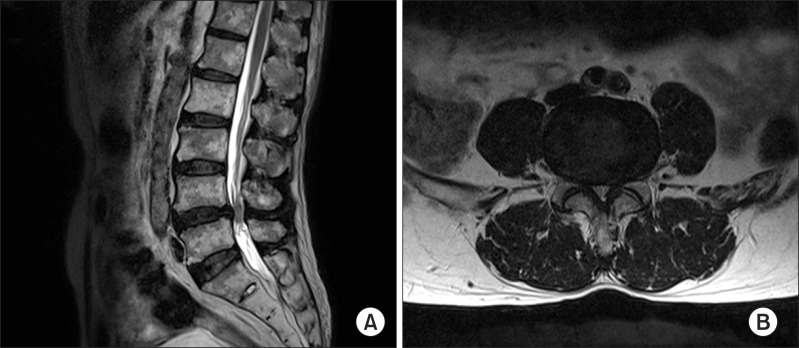
Fig. 3
Serial fluoroscopic images of percutaneous epidural adhesiolysis (PEA) using the inflatable balloon neuroplasty catheter. (A) Anteroposterior view verified before the procedure showing filling defects of contrast medium at the epidural space above the level of L5-S1 and both L5 intervertebral foramina. (B) Fluoroscopic view showing the inflatable balloon neuroplasty catheter placed in the L5 intervertebral foramen and the balloon filled with contrast medium. Foraminal stenosis is visualized by the degree of distortion of the balloon (arrow). (C) Decompression is performed along the intervertebral foramen by ballooning. (D) After decompression along the pass from the lateral recess to the intervertebral foramen, the contrast agent spread well. In addition, the contrast agent in the epidural space spread upward above the level of L5-S1.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download