Abstract
Glomus tumors are a rare, benign neoplasm and 75% exist in the subungual region. Extradigital glomus tumors are much more difficult to diagnose because of their atypical location and symptoms. Furthermore, if their symptoms are similar to neuropathic pain, the patient can suffer from misdirected treatment due to misdiagnosis. It is essential to perform careful evaluation of the lesion itself in order to reduce misdiagnosis. Ultrasonography is a useful, non-invasive method that can be easily performed in the pain clinic for local evaluation and diagnosis. We report a case of misdiagnosed glomus tumor in the thigh which was properly diagnosed after ultrasonography.
Glomus tumors are a rare, benign tumor accounting for less than 2% of soft tissue tumors [1]. 80% of the tumors are located in the upper extremities, and 75% of these are found in the subungual space [2]. Typical glomus tumors are relatively easy to diagnose due to the tumor's characteristic solitary lesion and classic triad of symptoms: pain, pinpoint tenderness, and hypersensitivity to cold [3]. However, extradigital glomus tumors are much more difficult to diagnose due to the absence of characteristic symptoms. Patients with extradigital glomus tumors can suffer greatly from misdiagnosis and improper treatment. Several diagnostic methods such as magnetic resonance imaging (MRI) and ultrasonography have been suggested to assist in rapid and accurate diagnosis. Ultrasonography can be readily used in the outpatient pain clinic without delay, providing help in evaluating the cause of localized pain. We report a case of a patient with a glomus tumor located in the anterior thigh, who was initially misdiagnosed with neuropathic pain but who was successfully treated after accurate diagnosis via ultrasonography.
A 65-year-old male patient was referred to our pain clinic due to pain in the left anterior thigh. The pain had begun 15 years earlier and had worsened following fine needle biopsy for the evaluation of a painful mass five years later. The nature of the pain was severe (score of 8 out of 10 on the visual analogue scale [VAS]) with constant dullness and paroxysmal lancinating pain. Clinical examination revealed severe tenderness at the biopsy site (VAS score 10 out of 10) and mild skin color change, loss of hair, decreased sweating, and static and dynamic allodynia and hyperalgesia in the left anterior thigh. The patient had been receiving treatment at another hospital where electromyogram, biopsy, and MRI results had been non-specific. Based on the clinical signs and symptoms, the patient had been diagnosed with complex regional pain syndrome (CRPS) type 1 and neuropathic pain. Previous treatments had included administration of non-steroidal anti-inflammatory drugs (NSAIDs), gabapentin, nortriptyline, opioids, and also several nerve blocks with little success. After arriving at our hospital, the patient received diverse interventional therapies during the next three months without significant results. Selective transforaminal epidural block (L1, L2 levels) and sympathetic ganglion block (L2 level) were effective in reducing pain 30% to 50% for only a day or two. Pulsed radiofrequency treatment of the L1 dorsal root ganglion also showed limited results. Although intravenous infusion of ketamine reduced pain for five days, the pain relief was only felt in the anterior thigh, while the tenderness in the biopsy site was not reduced.
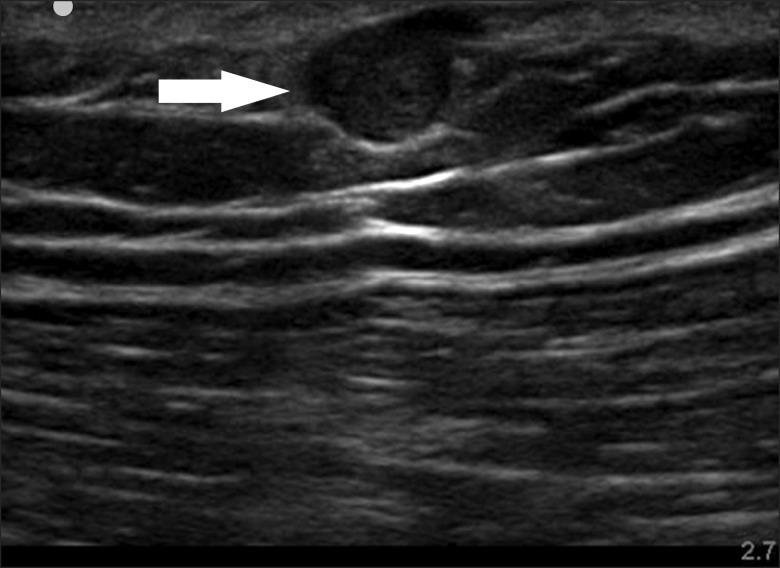
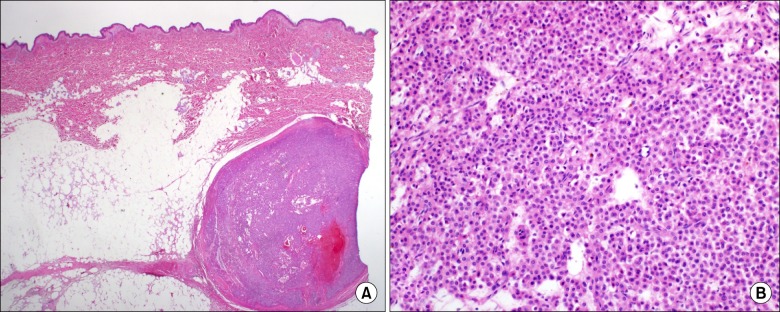
Further examination with ultrasonography at the biopsy site confirmed a round 0.8 × 0.6 cm2 sized hypoechoic cyst in the subcutaneous fat tissue (Fig. 1). The patient first received injection of 3 ml of 0.75% levobupivacaine around the cyst under ultrasonography guidance, but this did not provide any pain relief. One week later, 2 ml of 0.75% levobupivacaine was injected directly into the cyst. Although the patient complained of severe pain during penetration of the cyst, the pain was then almost completely reduced for 24 hours. Tenderness on the biopsy site was also reduced to a VAS score of 5 out of 10 (from 10 out of 10). The patient was transferred to the surgery department and was diagnosed with glomus tumor following excisional biopsy (Fig. 2). The pain was immediately reduced postoperatively and the patient has been pain free for six months.
The first description of a glomus tumor, as "painful subcutaneous tubercles", was made in 1812 by Wood [4]. Glomus tumor is a vascular tumor originating from the cutaneous neuromyoarterial glomus body. Glomus bodies are ubiquitous, arteriovenous anastomoses located between a preterminal arteriole and end efferent vein [5]. Found throughout the body, they are thermoregulatory contractile structures that regulate local skin blood flow [1]. Most glomus tumors are benign and small in size [6].
Although malignant glomus tumors are very rare, one should suspect malignancy if the tumors are found in a deep location and are larger than 2 cm or if they have histologic features of malignancy [7].
While glomus tumor may occur anywhere in the body, 75% originate in the hand, and most often in the subungual bed [2]. As extradigital glomus tumor is less common and the symptoms less specific, diagnosis is often delayed or even missed. Studies show that only 9% to 20% of patients were correctly diagnosed initially [8,9]. Schiefer et al. [9] have reported pain and localized tenderness in 86%, whereas only 2% presented with cold sensitivity in extradigital glomus tumors. In a study comparing 110 patients with digital and 42 patients with extradigital glomus tumor [8], the incidence of pain (82.4% vs. 70.3%) and cold sensitivity (25% vs. 0%) were significantly lower in extradigital tumor, whereas the incidence of tenderness did not differ significantly between extradigital (56.8%) and digital (77.8%) tumors. Our patient suffered from pain that mimicked neuropathic pain, which made us suspect neuropathic pain such as or peripheral neuropathy. There are only two similar reported cases where glomus tumor had been misdiagnosed as neuropathic pain. One was a tumor located at the abdominal wall and misdiagnosed as postherpetic neuralgia [10], and the other case was concomitant glomus tumor with CRPS in the hand [11]. Although relatively rare, it is possible that the pain physician can misdiagnose glomus tumor as neuropathic pain.
The mechanism of pain in glomus tumors has not been fully established, and several studies have suggested multiple mechanisms. The connective tissue capsules surrounding the tumors often contain bundles of myelinated and unmyelinated nerve fibers while the cytoplasm contains myofilaments resembling smooth muscle cells [12]. Unmyelinated nerve fibers have also been discovered in the tumor parenchyma [13]. Rohrich et al. [14] suggested that changes in temperature could lead to contraction of myofilaments in the glomus cells, resulting in an increase in intracapsular pressure that could be transmitted by the unmyelinated nerve fibers, leading to the perception of pain.
In the present case, pain was not induced during the injection of local anesthetics which should have increased intracystic pressure. Severe pain during needle contact and penetration into the cyst may be due to nerve fibers in the capsule, while inhibition of unmyelinated nerve fibers may have caused relief of pain following injection of local anesthetics in the cyst. We suspect that sensitization of unmyelinated nerve fibers in the cyst may cause tenderness and pain.
MRI is known to be the most sensitive imaging modality for digital glomus tumors and can also be beneficial in diagnosing extradigital glomus tumors [15]. The typical appearance of a glomus tumor on MRI is a decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images. Unfortunately, if the lesions are under 2-3 mm in diameter, results are likely to be false-negative [15].
Ultrasonography is a good alternative method for evaluation of extradigital glomus tumor in the outpatient clinic. Werner at al. [16] discussed the gray-scale sonographic findings of glomus tumor which were nonspecific when compared to other cystic lesions: well-described, ovoid, hypoechoic mass. Color Doppler imaging can be useful in the diagnosis of glomus tumor as it shows moderate to marked hypervascularity with arterial blood patterns [17].
Complete excisional biopsy is the most definitive method of both diagnosis and treatment. Inadequate excision may result in tumor recurrence within days to weeks, and symptoms appearing two to three years postoperatively may indicate multiple glomus tumors [9]. The recurrence rate after excision was reported to range from 2% to 10.5% in extradigital glomus tumors [8,9].
The pain physician must have knowledge of the diverse causes of neuropathic pain. Although they are quite rare, the physician must understand the nature of extradigital glomus tumors and provide adequate treatment following accurate diagnosis. We should always suspect additional causes such as glomus tumors when patients complain of pain despite diverse treatments. Ultrasonography can be useful for accurate diagnosis, especially in the outpatient clinic.
References
2. Kale SS, Rao VK, Bentz ML. Glomus tumor of the index finger. J Craniofac Surg. 2006; 17:801–804. PMID: 16877938.

3. Van Geertruyden J, Lorea P, Goldschmidt D, de Fontaine S, Schuind F, Kinnen L, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996; 21:257–260. PMID: 8732413.
4. Wood W. On painful subcutaneous tubercle. Edinb Med J. 1812; 8:283–291.
5. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008; 36:369–370. PMID: 18446863.

6. Chen SH, Chen YL, Cheng MH, Yeow KM, Chen HC, Wei FC. The use of ultrasonography in preoperative localization of digital glomus tumors. Plast Reconstr Surg. 2003; 112:115–119. PMID: 12832884.

7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001; 25:1–12. PMID: 11145243.
8. Lee DW, Yang JH, Chang S, Won CH, Lee MW, Choi JH, et al. Clinical and pathological characteristics of extradigital and digital glomus tumours: a retrospective comparative study. J Eur Acad Dermatol Venereol. 2011; 25:1392–1397. PMID: 21371130.

9. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006; 81:1337–1344. PMID: 17036559.

10. Kim YD, Son JS, Lee JW, Han YJ, Choi H, Jeong YJ. Extradigit glomus tumor causing abdominal pain -a case report-. Korean J Pain. 2012; 25:108–111. PMID: 22514779.

11. Jeong HJ, Kim CM, Yoon DM, Yoon KB. Concomitant glomus tumor with CRPS in the hand. Korean J Pain. 2013; 26:295–298. PMID: 23862005.

12. Carlstedt T, Lugnegård H. Glomus tumor in the hand. A clinical and morphological study. Acta Orthop Scand. 1983; 54:296–302. PMID: 6303040.

13. Kishimoto S, Nagatani H, Miyashita A, Kobayashi K. Immunohistochemical demonstration of substance P-containing nerve fibres in glomus tumours. Br J Dermatol. 1985; 113:213–218. PMID: 2411282.

14. Rohrich RJ, Hochstein LM, Millwee RH. Subungual glomus tumors: an algorithmic approach. Ann Plast Surg. 1994; 33:300–304. PMID: 7985967.

15. Glazebrook KN, Laundre BJ, Schiefer TK, Inwards CY. Imaging features of glomus tumors. Skeletal Radiol. 2011; 40:855–862. PMID: 21104079.

16. Werner JD, Wright CL, Iwenofu OH, Patil SB, Yuh WT. Unusual motion detected on real-time sonography inside a glomus tumor in the thigh. J Clin Ultrasound. 2013; 41:183–186. PMID: 22729971.

17. Park HJ, Jeon YH, Kim SS, Lee SM, Kim WT, Park NH, et al. Gray-scale and color Doppler sonographic appearances of nonsubungual soft-tissue glomus tumors. J Clin Ultrasound. 2011; 39:305–309. PMID: 21520136.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download