Abstract
More than 80% of cancer patients experience cancer pain. Among them, more than 50% experience moderate to severe pain. To control cancer pain, a variety of methods have been used, including medications and nerve blocks. In some patients, however, it is impossible to perform nerve blocks due to caner metastasis into the epidural space, while in other patients, opioid dose escalation is impossible due to opioid side effects; thus, cancer pain management is difficult. Scrambler therapy is a novel approach for pain control that uses EKG-like pads, which are applied above and below the site of pain. Scrambler therapy synthesizes 16 different types of nerve action potentials that provide "non-pain" information via cutaneous nerves. The advantages of this treatment are that it is non-invasive and safe and has no significant side effects. In this case series, we report the treatment results of using scrambler therapy in three cancer patients with intractable pain.
Go to : 
The death rate of cancer patients is continually decreasing due to the development of new chemotherapy agents and advancements in radiation therapy and surgical methods. For cancer patients with increased life expectancies, quality of life has become an important issue. Among the various factors that determine quality of life for such patients, cancer pain is the most important. Medication therapy-including NSAIDs, opioids, antidepressants, and antiepileptic drugs-as well as nerve blocks and patient-controlled analgesia (PCA) are provided as treatment for cancer pain. Despite the availability of various treatments, however, the existing literature reveals the difficulty of controlling cancer pain. Breivik et al. [1] reported that in a study of 5,084 cancer patients, 56% had cancer pain of a moderate or worse degree, and only 41% of the patients were using appropriate opioids. In addition, in the reports of Von Roenn et al. [2] and Zenz et al. [3], 40-70% of patients with cancer pain were receiving inappropriate pain treatment. Insufficient treatment for cancer pain arises from several factors that interfere with pain treatment; such factors include fear and aversion of patients and medical staff toward the use of opioids because of the possibility of opioid abuse and addiction, insufficient palliative care programs, lack of experience and inexperienced use of opioids by medical staff, and complications arising from the use of opioids. In addition, medical staff may perform inappropriate pain control due to their lack of understanding of the pathophysiologic characteristics of cancer pain, such as neuropathic pain, breakthrough pain, and cancer-induced bone pain [4]. Additionally, performing nerve blocks can be dangerous in patients who have coagulopathy due to underlying diseases such as leptomeningeal seeding, local infection of nerve block area, cancer metastasis, liver failure, and renal failure, or in patients who are undergoing anticoagulant therapy to treat underlying diseases [5,6]. Opioids are used as the primary medication to control cancer pain, but pain control is difficult in some patients due to the various adverse effects of opioids (nausea, vomiting, constipation, etc.). Due to this range of problems, control of cancer pain is not an easy area of pain management.
The recently introduced scrambler therapy could be a good choice for patients with cancer pain who are having difficulty with pain control. The advantage of scrambler therapy is that it works by attaching electrodes to the skin, as in transcutaneous electrical nerve stimulation (TENS); hence, it is non-invasive and has no side effects. Scrambler therapy is an innovative treatment method that attaches electrodes bilaterally outside the area of pain (where pain is not felt) and blocks the pain signals of the painful area by conveying "non-pain" information via electrical stimulation to the central nerve system [7]. With regard to the treatment effect of scrambler therapy, no research has yet been conducted on various types of cancer pain, although Smith et al. [8] published a study on the effect of scrambler therapy in chemotherapy-induced peripheral neuropathy. According to their results, scrambler therapy had a 64% pain reduction effect. In our case reports, scrambler therapy was applied to patients with cancer pain who had difficulty controlling pain with other treatment methods. As good results were obtained, we report the results herein.
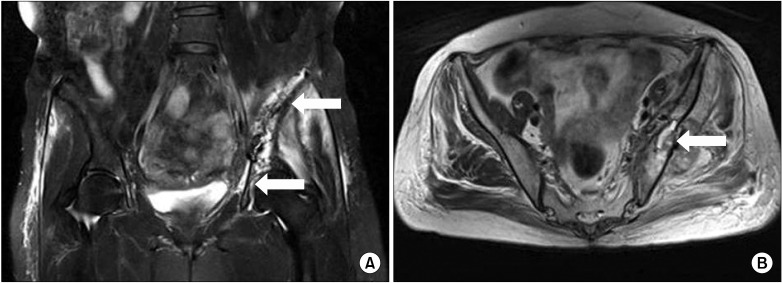
A 55-year-old female patient with a height of 151 cm and a weight of 55 kg was referred from the hematooncology clinic for left buttock pain caused by right infiltrative ductal breast cancer with left pelvic bone metastasis. The degree of pain on the 1-10 numeric rating scale (NRS) was 8, and it was characterized as a spontaneous tingling and ripping pain. She had received breast conserving surgery for right breast cancer 2 years before, but metastasis to the left pelvic bone had been discovered in an MRI one year before. Chemotherapy with adriamycin, cyclophosphamide, and taxol had been performed 6 times, and radiation therapy had been performed 8 times, but there had been no reduction in tumor size. Metastasis had occurred in the right acetabulum. Hence, palliative chemotherapy of Gemzar and Navelbine had been performed 6 times, and radiation therapy had been performed 6 times. At the time of referral, the patient was only scheduled for palliative care. The patient was having difficulty walking due to the pain in the pelvic area. In the MRI and bone scan of the pelvic area performed before referral, bone metastasis was observed in the left ilium, left ischium, and right acetabulum, in addition to a pathologic fracture in the left iliac wing (Fig. 1). At the time of referral, the patient was administered with fentanyl patch 100 µg/hr, oxycodone IR 10 mg bid, gabapentin 200 mg tid, and carbamazepine 200 mg bid for pain control; however, she was continuously complaining of severe pain (NRS 8/10) that worsened when walking. During hospitalization, fluoroscopically guided caudal epidural injection of 0.125% Chirocaine™ (levobupivacaine hydrochloride, Abbott Korea, Korea) 10 ml and Triam™ (triamcinolone acetonide, Shin Poong Pharm, Korea) 20 mg was performed twice with a one-week interval. However, the pain alleviation effect did not continue for more than one day after the procedure. Hence, scrambler therapy (MC5-A Calmare®) was planned. Because the patient's pain was located at the S1-2 dermatome of the left buttocks, the scrambler electrodes were attached at 4 normal sensory areas above and below the painful area of the S1-2 dermatome for treatment. During the scrambler treatment, the NRS score decreased to 0. The NRS score was maintained at 3 in the ward on the day of treatment. The patient was discharged after 10 sessions of scrambler therapy. During the 10 sessions, the dermatome location of pain did not change, but the area of pain slowly contracted; hence, the painful area of the patient was consulted daily before attaching the electrodes. Pain medications were continually administered without a change in dosage during the scrambler therapy. The pain alleviation effect through scrambler therapy continued for 2 months; on the third month, the pain recurred in the same area. The patient was re-hospitalized, and a second round of scrambler therapy was started. During treatment, the NRS score decreased from 7/10 to 0/10 on the first day, and this was maintained for 3-4 hours after treatment. Subsequently, the NRS score remained at 3.5/10 for 2 months, and pain medication was consistently given without a change in dosage during this period.
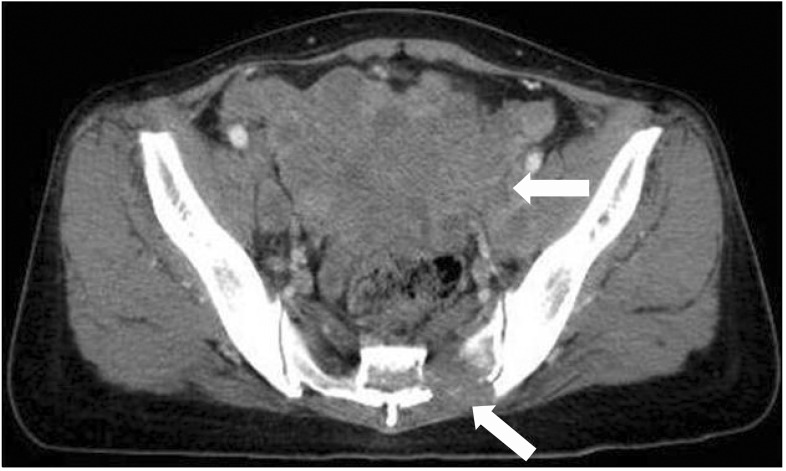
A 49-year-old female patient with a height of 152 cm and a weight of 45 kg was referred by her Obstetrics and gynaecology for bilateral sacral area pain caused by uteuterine sarcoma and bilateral sacral bone metastasis. The patient complained of a spontaneous, throbbing pain with an NRS score of 8/10, and it worsened with changes in position or movement. Total laparoscopic hysterectomy had been performed on the patient for uterine sarcoma one year before. Because discomfort in the lower abdomen and symptoms of tenesmus had continued after surgery, a CT scan of the abdomen had been performed one month after surgery. In the scan, a heterogeneously enhancing mass lesion of 14.6 × 7.7 cm in the pelvic cavity and numerous lymph node enlargements in the abdominal cavity had been observed, as well as a soft mass lesion of 3.7 × 2.9 cm in the left pelvic bone and a lesion of 2.5 cm2 in the right pelvic bone (Fig. 2). Doxorubicin and cisplatin chemotherapy had been started and further radiation therapy scheduled. At the time of referral, the patient was taking oxycodone PR 20 mg bid, as well as oxycodone IR 5 mg when pain increased, as prescribed by the referring department. However, severe opioid-induced constipation developed, and the patient was refusing an increase in opioids despite the increase in pain level. Thus, scrambler therapy was planned for the patient. As the patient's area of pain was located on the bilateral S3-5dermatome, the scrambler electrodes were attached to 6 normal sensory areas to the left and right of the S3-5dermatome pain area for treatment. Scrambler therapy was performed 10 times for 40 minutes once every day, and oral pain medication was continually administered without a change in dosage. From the first day of treatment, the NRS score of the affected area decreased from NRS 8/10 to 0/10, and the NRS score at home after the procedure decreased to 2.5/10; this was maintained for approximately 2 weeks after treatment. During the 10 sessions of scrambler therapy, the dermatome location of pain did not change, but the painful area slowly contracted; thus, the patient's area of pain was consulted daily before attaching the electrodes. The pain medications were continually administered without a change in dosage during the scrambler therapy. One week after completion of scrambler therapy, the patient developed symptoms of diarrhea as she started radiation therapy. In addition, the pain in the sacral area worsened. The patient was re-hospitalized, as she wanted a second round of scrambler therapy. There was no pain during the subsequent scrambler therapy, and the chronic diarrhea improved. Her pain at home was maintained at around NRS 3/10 for one month. Afterwards, the patient was transferred to another hospital near her hometown.
A 56-year-old male patient with a height of 166.6 cm and a weight of 59.9 kg was referred from the gastroenterology department for right chest pain caused by hepatocellular carcinoma and right chest fifth rib metastasis. The degree of pain was NRS 6/10, and it was characterized as a spontaneous splitting pain. Transarterial chemoembolization (TACE) chemotherapy had been performed on the patient for liver cancer for one year. Five months before visiting the cancer pain unit at our hospital, metastasis to the right chest fifth rib had been discovered, and the patient had received radiation therapy. In the subsequent MRI and bone scan of the thoracic vertebrae, right fifth rib metastasis with extraosseous mass formation had been observed. The patient had the underlying diseases of hepatitis B and liver cirrhosis and, at the time of referral, had been using Ultracet Tab™ (acetaminophen 325 mg tramadol hydrochloride 37.5 mg, Janssen Korea LTD, Korea) 2 tab tid for pain control. However, the right chest pain had gradually worsened and been causing sleep disturbance. Hence, a thoracic epidural injection had been performed during hospitalization, but 3 days after the procedure, the pain had worsened, with the NRS score increasing again to 6/10 from 2/10; thus scrambler therapy (MC5-A Calmare®) was planned for the patient. The patient complained of chest pain in the right fifth rib area, so the scrambler electrodes were attached to 2 normal sensory areas to the left and right of the right fifth rib pain area for treatment. As the 10 sessions of treatment progressed, it was clear that the pain area was gradually contracting, and the location of the electrodes was adjusted accordingly for treatment. After 2 sessions of scrambler therapy, the NRS score decreased to 2/10, and the treatment effect continued until the next morning. The administration of Ultracet Tab™ was stopped accordingly. After 10 sessions of scrambler therapy, the NRS score has been maintained at 2/10, and the pain area maintained at about 80% reduced state, for 2 months.
Go to : 
In the above cases, scrambler therapy demonstrated effective treatment in patients complaining of severe cancer pain but unable to experience relief from nerve blocks or medication therapy. The patient in case 1 had received fluoroscopically guided caudal block for pain in the left buttock caused by right breast cancer and metastasis in the left pelvic area, but the pain alleviation effect was minimal. After scrambler therapy, however, the NRS score was reduced and maintained at 3.5/10 from 7/10. The patient in case 2 was suffering from bilateral sacral pain from uterine carcinoma and bilateral pelvic area metastasis, and she could not increase medication due to side effects from opioids. However, after scrambler therapy, the NRS score was reduced and maintained at 3/10 from 8/10. The patient in case 3 had received a thoracic epidural injection for right chest pain caused by hepatocellular carcinoma and metastasis to the right chest fifth rib, but the pain alleviation effect did not continue for more than 3 days. Hence, the patient underwent scrambler therapy, and the NRS score was reduced and maintained at 2/10 from 6/10.
The mechanism of scrambler therapy has not been clearly revealed, similar to other electrical stimulation therapies such as TENS or spinal cord stimulation (SCS). Marineo, the developer of scrambler therapy, suggested the following mechanism: Scrambler therapy provides "no-pain" information to the periphery sensory nerve receptors through attached electrode patches, and this is conveyed to the central nervous system and remembered by the system to relieve patients' pain. This electrical stimulus is conducted through C-fiber and Aδ fiber, which usually convey pain, but it is not a method of simply stimulating the periphery pain nerves that cause pain. It is also different from dulling the senses of the patient, so that he or she can still feel normal stimulation on the treated area after scrambler therapy. The effect of scrambler therapy appears within 10 seconds of starting treatment, and pain alleviation is maintained continuously for several days or several months after completion of treatment. The mechanism signifies that remodulation occurs in the periphery and central nervous system or the calcium channels of the synapses, which become the main target for treating neuropathic pain. Finally, the patient feels the information conveyed by scrambler electrodes by the entire dermatome where the electrodes are attached rather than only the area where the electrodes are attached; thus, it is evident that "no-pain" information is conveyed through the dermatome [7]. However, no research results have yet supported these findings in the present literature.
Procedures for scrambler therapy start from first clearly defining the pain area. Next, electrodes are attached to the areas proximal and distal to the pain area. Here, it is recommended that the electrodes are attached along the dermatome of the pain area, and they should be positioned in areas where there is no pain. Subsequently, electrical stimulus is applied and the intensity is increased gradually; the intensity of the electrodes is set to the maximum value at which the patient does not feel discomfort. In this stage, the electrical stimulus conveys 16 types of signals similar to the action potential conveyed through the nervous system. In the initial treatment, the 16 types of action potential, frequency from 43 to 52 Hz, duration of electrical stimulus from 0.7 to 10 seconds, and amplitude are dynamically adjusted to find the optimal "no-pain" information appropriate for the patient [7]. Through these processes, the patient feels his or her pain disappearing within 10 seconds of starting therapy. If there is no pain relief, the procedures are started again after moving the electrodes to a different area. In addition, if there are areas where pain remains, more electrodes are attached to alleviate the remaining pain. Scrambler therapy is performed for 40 minutes per session, and the treatment should be performed every day if possible.
To the present, there have been 4 papers published regarding the treatment effect of scrambler therapy. The first study was conducted in 11 patients with pancreatic cancer; as a result of scrambler therapy, 9 patients were able to discontinue drug treatment [9]. The second study was conducted in 226 patients complaining of non-cancerous neuropathic pain where medication had no effect; with scrambler therapy, 80% of patients were improved (improvement of 50% or more), 10% showed partial improvement (improvement of 25-49%), and 10% had no improvement (improvement of less than 24% or Visual Analogue Scale (VAS) score > 3) [10]. The third study reported the effect of scrambler therapy in 16 patients complaining of peripheral neuralgia caused by chemotherapy. The pain completely disappeared in 4 out of the 16 patients, and 64% of the pain improved in most patients. The authors of this study concluded that scrambler therapy is effective in pain alleviation without side effects in patients with peripheral neuralgia caused by chemotherapy [8]. In contrast to the above three, the most recently published paper was a randomized controlled trial with a control group [7]. This study compared treatment results between a drug therapy group and a scrambler therapy group in patients with chronic neuropathic pain; one month after treatment, the NRS score in the control group decreased 28%, from 8.1/10 to 5.8/10, while the scrambler therapy group decreased 91%, from 8/10 to 0.7/10 (P < 0.0001). The pain scores of the control group 2 and 3 months after starting treatment were 5.7/10 and 5.9/10, respectively, while the scrambler treatment group had scores of 1.4/10 and 2/10 (P < 0.0001) [7]. The patients in our case study also showed a pain alleviation effect of 70% or more, which is similar to existing papers. The patient in case 3 showed a reduction in the degree and area of pain of up to 80%. However, the patients in cases 1 and 2 showed a recurrence of pain with the passage of time, and this is considered to be the result of continuous tissue destruction of cancerous tissue and subsequent pain stimulus. Precedent reports suggested that dosage of analgesics can be significantly reduced when there is improvement from scrambler therapy; in our cases, however, the patients had mainly complained of local pain caused by bone metastasis of cancerous pain, mostly accompanied by intermittent pain in the cancer area. We also considered the fact that excessive reduction of analgesics is not recommended in terminal cancer patients. Therefore, we did not promote excessive reduction of analgesics during and after scrambler treatment.
Other methods for treating chronic pain using electrical stimulus are SCS and TENS. Many hypotheses have been put forward to explain the treatment mechanism of SCS and TENS, such as supraspinal processes, modulation of descending inhibitor pathways, peripheral release of calcitonin, increased gate control for pain threshold, reduction of windup phenomenon, and reduction in impulses from damaged nerves [8,11,12]. Additionally, SCS reduces the stimulation of damaged nerves and is known to reduce psychological maladaptation for pain [13]. However, similar to scrambler therapy, the precise treatment mechanism has not been revealed for either of these methods.
Scrambler therapy is quite similar to existing TENS in that it treats pain by applying electrical stimulus from electrodes attached to the skin. However, scrambler therapy shows differences with TENS in the method of electrical stimulus and treatment effect. First, TENS positions the electrodes on the area of pain, while scrambler therapy positions the electrodes on normal sensory areas surrounding the area of pain. Second, TENS has shown a limited effect in patients with postherpetic neuralgia (PHN), and the treatment effect also disappears within a few hours [14]. In contrast, scrambler therapy has shown great results in the pain treatment of PHN patients [7]. Third, TENS conveys unchanging on-off biphasic electric waves, whereas scrambler therapy provides 16 nonlinear waveforms that change continuously [15].
In addition, when the treatment effects of SCS are examined, patients with complex regional pain syndrome type I showed a VAS score reduction from 10/10 to 2/10 when SCS was performed [16], while PHN patients showed a VAS median value reduction from 9/10 (interquartiles 7.75-10) to 1/10 (interquartiles 1.0-2.75) [17]. In addition, when compared with existing drug therapy, SCS reduces pain by 50% or more in failed back surgery syndrome patients, and the effect continued for 24 months [18]. In this way, SCS is an effective method for treating chronic pain, but it is invasive and expensive. There is also a high risk of complications, including one report of a 38% occurrence of hardware-related complications [19]. Scrambler therapy has also proven to be an effective treatment method for patients complaining of various types of chronic pain and peripheral neuralgia caused by chemotherapy [20]. This case study has also demonstrated a good treatment effect for pain caused by bone metastasis of cancer. However, the severity and incidence of complications cannot be compared with SCS, as the only complication from scrambler therapy is skin irritation in the area of electrode attachment.
The factors that determine response to scrambler therapy have not yet been revealed. Ricci et al. [20] reported the effects of scrambler therapy on 40 patients with peripheral neuralgia caused by chemotherapy and 33 non- cancerous pain patients. Two weeks after 10 sessions of scrambler therapy, a response rate of 71% was observed in the peripheral neuralgia patients (64% complete response where pain was reduced 50% or more, 7% partial response where pain was reduced 25-49%), and an 85% response rate was seen in non-cancerous patients (70% complete response, 15% partial response). There were no statistical differences between the improved group and the non-improved group in factors that may decide response rate to scrambler therapy, such as nociceptive/neuropathic characteristics of pain and degree of improvement, duration of pain (1-3 months or 3 months or more), and characteristics of pain (continuous pain/continuous plus breakthrough pain/intermittent pain) [20]. It is necessary to research the factors that influence response to scrambler therapy through large-scale research in the future.
The authors were able to obtain a satisfactory effect by administering scrambler therapy in patients with cancerous pain caused by bone metastasis of cancer cells and in whom pain control through palliative treatment methods had proven difficult. Scrambler therapy is non-invasive, has no complications, causes minimal discomfort during treatment, and is similar or superior to other existing treatments in effect and duration. However, there is insufficient comparative research regarding the effect of scrambler therapy for various types of pain. Therefore, validation is needed. More research is also necessary regarding the factors that influence response to the treatment.
Go to : 
References
1. Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJ, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009; 20:1420–1433. PMID: 19244085.

2. Von Roenn JH, Cleeland CS, Gonin R, Hatfield AK, Pandya KJ. Physician attitudes and practice in cancer pain management. A survey from the Eastern Cooperative Oncology Group. Ann Intern Med. 1993; 119:121–126. PMID: 8099769.

3. Zenz M, Zenz T, Tryba M, Strumpf M. Severe undertreatment of cancer pain: a 3-year survey of the German situation. J Pain Symptom Manage. 1995; 10:187–191. PMID: 7629412.

5. Li SL, Wang DX, Ma D. Epidural hematoma after neuraxial blockade: a retrospective report from China. Anesth Analg. 2010; 111:1322–1324. PMID: 20705781.
6. Mertes N, Goeters C, Bantel C, Gullotta F, Van Aken H. Brain death during anesthesia due to undiagnosed meningeal carcinomatosis of gastric adenocarcinoma. Anesthesiology. 1999; 90:630–631. PMID: 9952174.

7. Marineo G, Iorno V, Gandini C, Moschini V, Smith TJ. Scrambler therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: results of a pilot, randomized, controlled trial. J Pain Symptom Manage. 2012; 43:87–95. PMID: 21763099.

8. Smith TJ, Coyne PJ, Parker GL, Dodson P, Ramakrishnan V. Pilot trial of a patient-specific cutaneous electrostimulation device (MC5-A Calmare®) for chemotherapy-induced peripheral neuropathy. J Pain Symptom Manage. 2010; 40:883–891. PMID: 20813492.

9. Marineo G. Untreatable pain resulting from abdominal cancer: new hope from biophysics? JOP. 2003; 4:1–10. PMID: 12555009.
10. Sabato AF, Marineo G, Gatti A. Scrambler therapy. Minerva Anestesiol. 2005; 71:479–482. PMID: 16012423.
11. de Leon-Casasola OA. Spinal cord and peripheral nerve stimulation techniques for neuropathic pain. J Pain Symptom Manage. 2009; 38:S28–S38. PMID: 19671469.

12. Foletti A, Durrer A, Buchser E. Neurostimulation technology for the treatment of chronic pain: a focus on spinal cord stimulation. Expert Rev Med Devices. 2007; 4:201–214. PMID: 17359225.

13. Jensen MP. A neuropsychological model of pain: research and clinical implications. J Pain. 2010; 11:2–12. PMID: 19595637.

14. Nathan PW, Wall PD. Treatment of post-herpetic neuralgia by prolonged electric stimulation. Br Med J. 1974; 3:645–647. PMID: 4425789.

15. Niv D, Maltsman-Tseikhin A, Lang E. Postherpetic neuralgia: what do we know and where are we heading? Pain Physician. 2004; 7:239–247. PMID: 16868598.
16. Harke H, Gretenkort P, Ladleif HU, Rahman S. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective clinical study. Eur J Pain. 2005; 9:363–373. PMID: 15979016.

17. Harke H, Gretenkort P, Ladleif HU, Koester P, Rahman S. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg. 2002; 94:694–700. PMID: 11867400.

18. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007; 132:179–188. PMID: 17845835.

19. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011; 11:148–153. PMID: 21371254.

20. Ricci M, Pirotti S, Scarpi E, Burgio M, Maltoni M, Sansoni E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare® device. Support Care Cancer. 2012; 20:405–412. PMID: 21394458.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download