Abstract
Zoster sine herpete (ZSH) is difficult to diagnosis during an acute period due to the absence of the characteristic zosteriform dermatomal rash; therefore, progression to postherpetic neuralgia is more common than typical zoster. In addition, misdiagnosis of other neuropathic pain as ZSH is common in clinical situations. Here, we report a case of spinal arteriovenous malformation that mimics ZSH. This is a rare condition; therefore, high clinical suspicion for a correct diagnosis and proper examination are not easy. However, early diagnosis and definitive treatment are essential to prevent neurologic deficit and mortality.
Go to : 
Herpes zoster (HZ) is a clinical syndrome where topical skin irritation, pain and paresthesia occur along the distribution of the dermatome, due to varicella zoster virus entering through the sensory nerve of the skin and is dormant in the dorsal root ganglia or the cerebral ganglia until it is reactivated when the immune system of the body becomes weak. When pain persists for 1, 3, 4, or 6 months or more after developing a rash, it is called postherpetic neuralgia (PHN) [1].
There are rare cases of HZ which do not form vesicular eruption on the skin. Weber [2] calls this zoster sine herpete (ZSH). Rather than early diagnosis, in most cases, it is diagnosed as ZSH when the patient complains of chronic continuing dermatomal neuropathic pain. Before diagnosis, it is important to differentiate from other diseases that can cause dermatomal neuropathic pain with an accurate physical examination [3].
This case will examine spinal arteriovenous malformation (AVM), which was misdiagnosed as ZSH.
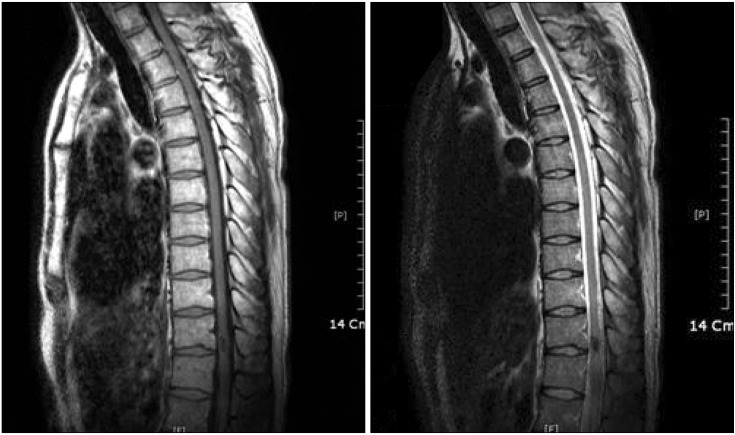
A 36-year-old man with an itching and tingling sensation in the left L2 dermatome area for 3 months was referred to our clinic for pain management. The patient did not have significant past medical history, and before coming to the clinic, he had been treated with gabapentin 600 mg 3 times a day by a dermatologist but the symptoms were not improved. There were no unusual finding in the biopsy other than subcorneal pustules, hyperkeratosis and parakeratosis; therefore, he was referred to our department for pain control. The character was continuous tingling and itching, and it was aggravated when humid. He was also complaining of allodynia and paresthesia, and his visual analogue scale (VAS) was 2 out of 10 (0 = no pain, 10 = worst possible pain). His motor function was intact and there was no sensory loss. His knee jerk reflex was increased (3+/3+) thus a thoraco-lumbar spine magnetic resonance imaging (T-L MRI) was performed due to suspicion of an upper motor neuron (UMN) lesion. In the MRI, an intramedullary lesion 1.2 cm in size was found in the dorsal subpial surface of the T11-12 level, and this was diagnosed as type II AVM in the T11/12 level of the spinal cord (Fig. 1).
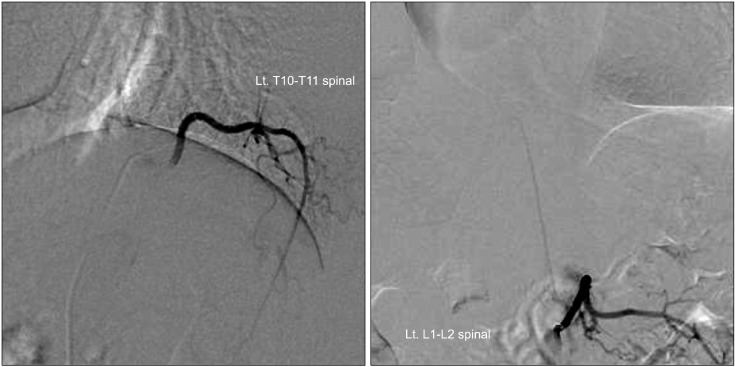
A spinal angiography was performed on the patient and the results revealed that the lesion was a spinal cord AVM supplied by the anterior spinal artery from the left T9 intercostal artery. It was also contrasted from the left L1 lumbar artery (Artery of Adamkiewicz) (Fig. 2).
The patient was transferred to another hospital for removal of AVM with cyber knife, and after the operation, the symptoms were improved.
Go to : 
HZ is usually diagnosed by characteristic clinical symptoms including dermatomal rash. Laboratory tests are the Tzank smear, a serological test such as the indirect fluorescent antibody method, and detection through molecular biology or immunology such as tissue cultures [4].
A dermatomal rash is not present in ZSH so it is difficult to suspect HZ, and hence, serological, immunological, or molecular biological tests are not performed during the acute phase. Only when the pain continues chronically are many cases diagnosed by the characteristics of pain. However, when a patient complains of continuing dermatomal neuropathic pain, a detailed history taking and accurate physical examination should be done to differentiate ZSH from sciatica, collapsed intervertebral disc, spinal stenosis, tumor, angina, renal colic, cholecystitis, costochondritis, intercostal neuralgia, entrapment syndrome, pleurodynia, myofascial pain syndrome, and other neuropathic pain before diagnosis [3].
In this case, the patient complained of tingling pain with a VAS score of 2 and accompanying itching, paresthesia, and allodynia in the left L2 dermatome for 3 months. During the physical examination, motor and sensory function was intact but the knee jerk reflex was increased to 3+ on both sides. Hence, an UMN lesion above the L2 level was suspected, an AVM in the T11/12 level was discovered in the T-L MRI. The patient received the appropriate treatment with cyber knife.
The UMN is the pathway from the brain and brainstem to the anterior horn of the spinal cord. The lower motor neuron (LMN) is the pathway from the anterior horn of the spinal cord to the skeletal muscles. In this case, only the knee jerk reflex was hyperactive with L2 dermatomal pain without any other general symptoms or symptoms in the upper extremity. Therefore, a spinal cord lesion in a level higher than the L2 level was anticipated, and a T-L MRI was performed to diagnose the AVM. Other symptoms that suggest an UMN lesion are weakness, paralysis, spasticity, and muscle atrophy, but these symptoms were not observed in our patient. Only the hyperactivity of the knee jerk reflex was observed, but if the Babinski response (the most sensitive indicator of the UMN) had been checked, it would have been more helpful in making the diagnosis [5,6].
AVM is the abnormal development of blood vessels which occurs during the 4th to 8th week of embryonic development when blood vessels are differentiated into arteries, veins, and capillaries [7]. Normally arteries and veins are connected by capillaries, but there is no true capillary bed in AVM; thus over time, a tangle is formed in the blood vessel connecting the artery and vein. Therefore, oxygenated arterial blood is shunted and supplied directly into the venous system which can cause temporary or permanent ischemia in the surrounding tissue. AVM can develop anywhere in the body and there can be various manifestations of symptoms according to the location and blood flow. Usually, it develops in the limbs, neck, and face, and it can cause pain, congestive heart failure, and hypotension from vascular congestion and so on [8].
Cerebral AVM can cause seizure or severe headaches, but mostly there are no symptoms. Spinal AVM has a prevalence of approximately 10% for cerebral AVM and less than 5% for an intraspinal mass. As in this case, it usually occurs in middle-aged men in the lower thoracic or lumbar region. Typical early symptoms are dermatomal lancinating pain, weakness/paralysis, numbness, and paresthesia [9-11].
Spinal AVM are classified by 4 types according to its form. In this case, glomus-type (type 2) with a tightly compacted group of arterial and venous vessels inside a short segment of the spinal cord, and usually, it is supplied by one artery. Neurologic symptoms are worsened according to the increased venous pressure [12,13].
Spinal AVM is very rare with a prevalence of less than 1%, shows various clinical symptoms, and is difficult to differentiate from other diseases such as tumors, herniated nucleus pulposus, multiple sclerosis, meningovascular syphilis, tuberculosis, and transverse myelitis. However subarachnoid hemorrhage or spinal cord hemorrhage due to AVM rupture and the subsequent acute neurologic deficit are increasing by 2-3% every year, and the mortality at first hemorrhage reaches 10-30%; therefore, an accurate differential diagnosis is very important [11,14,15]. Additionally, in this case, the AVM was discovered in the MRI, but there are many cases which cannot be detected by MRI thus an angiography must be performed for diagnosis.
In this case, spinal AVM was accurately diagnosed through a detailed neurologic examination, and the appropriate treatment was given. Therefore, when a patient complains of dermatomal neuropathic pain without a rash, a detailed and accurate physical examination should be performed before diagnosing ZSH, and laboratory tests should be performed for the acute phase to differentiate it from other diseases that cause neuropathic pain.
Go to : 
References
1. Straus SE, Oxman MN, Schmader KE. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Varicella and herpes zoster. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;p. 1885–1898.
2. Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000; 55:708–710. PMID: 10980741.

3. Rajbala T, Joel LK, Robert HD. Fishman SM, Ballantyne JC, Rathmell JP, editors. Herpes zoster and postherpetic neuralgia. Bonica's management of pain. 2010. 4th ed. Philadelphia: Lippincott Williams & Wilkins;p. 338–357.
4. Cohen PR. Tests for detecting herpes simplex virus and varicella-zoster virus infections. Dermatol Clin. 1994; 12:51–68. PMID: 8143385.

5. Murray B, Mitsumoto H. Daroff RB, Fenichel GB, Jankovic J, Mazziotta JC, editors. Disorders of upper and lower motor neurons. Bradley's neurology in clinical practice. 2012. 6th ed. Philadelphia: Elsevier;p. 1855–1886.

6. Lewis SL. Weiner WJ, Goetz CG, editors. An approach to neurologic symptoms. Neurology for the non-neurologist. 2004. 5th ed. Philadelphia: Lippincott Williams & Wilkins;p. 21–33.
7. Fisher WS 3rd. Wilkins RH, Rengachary SS, editors. Concomitant intracranial aneurysms and arteriovenous malformations. Neurosurgery. 1996. 2nd ed. New York: McGraw-Hill;p. 2429–2431.
8. Neifeld JP, Doppman JL, Chreitien PB. Congenital pelvic arteriovenous fistulas: report of a case and review of the literature. J Urol. 1975; 114:648–652. PMID: 1235402.

9. Gennuso R, Zappulla RA, Strenger SW. A localized lumbar spinal root arteriovenous malformation presenting with radicular signs and symptoms. Spine (Phila Pa 1976). 1989; 14:543–546. PMID: 2727801.

10. Elhammady MS, Wolfe SQ. Deshaies EM, Eddleman CS, Boulos AS, editors. Aziz-Sultan MA. Spinal arteriovenous malformations. Handbook of neuroendovascular surgery. 2012. 1st ed. New York: Thieme Medical Publishers;p. 375–387.
11. Geldmacher DS. Daroff RB, Fenichel GB, Jankovic J, Mazziotta JC, editors. Vascular diseases of the nervous system: spinal cord vascular disease. Bradley's neurology in clinical practice. 2012. 6th ed. Philadelphia: Elsevier;p. 1095–1102.
12. Riina HA, Soni D, Stieg PE. Batjer HH, Loftus CM, editors. Surgical treatment of spinal arteriovenous malformations. Textbook of neurological surgery: principles and practices. 2003. Philadelphia: Lippincott Williams & Wilkins;p. 2569–2578.
13. Heros RC, Debrun GM, Ojemann RG, Lasjaunias PL, Naessens PJ. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986; 64:134–139. PMID: 3941336.
14. Caroscio JT, Brannan T, Budabin M, Huang YP, Yahr MD. Subarachnoid hemorrhage secondary to spinal arteriovenous malformation and aneurysm. Report of a case and review of the literature. Arch Neurol. 1980; 37:101–103. PMID: 7356401.

15. Smith BS, Penka CF, Erickson LS, Matsuo F. Subarachnoid hemorrhage due to anterior spinal artery aneurysm. Neurosurgery. 1986; 18:217–219. PMID: 3960303.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download