Abstract
Complex regional pain syndrome secondary to brachial plexus injury is often severe, debilitating and difficult to manage. Percuteneous radiofrequency sympathectomy is a relatively new technique, which has shown promising results in various chronic pain disorders. We present four consecutive patients with complex regional pain syndrome secondary to brachial plexus injury for more than 6 months duration, who had undergone percutaneous T2 and T3 radiofrequency sympathectomy after a diagnostic block. All four patients experienced minimal pain relief with conservative treatment and stellate ganglion blockade. An acceptable 6 month pain relief was achieved in all 4 patients where pain score remained less than 50% than that of initial score and all oral analgesics were able to be tapered down. There were no complications attributed to this procedure were reported. From this case series, percutaneous T2 and T3 radiofrequency sympathectomy might play a significant role in multi-modal approach of CRPS management.
Go to : 
Traumatic brachial plexus lesions are one of the most common forms of plexus injury resulting from high-impact trauma in young adult males, as is often observed following a motor vehicle accident or industrial injury. Chronic neuropathic pain and subsequent development of complex regional pain syndrome (CRPS) type II are usual following such injuries of the brachial plexus [1]. Continuous pain following brachial plexus injury is particularly difficult to manage. Interventions in the form of sympathectomy might play a role in multimodal treatment in cases in which sympathetically mediated pain is a major component of the pain in CRPS [2]. Stellate ganglion blockade has always been the initial treatment option for upper limb CRPS should conservative management fail. However, repeated reports have shown that stellate ganglion blockade lacks promise in terms of efficacy in the management of upper limb CRPS [3]. When the sympathetic innervation of the upper limb is better defined, upper thoracic sympathectomy with higher efficacy and fewer complications in controlling pain of the upper limbs could be achieved [4]. Percutaneous T2 and T3 radiofrequency (RF) sympathectomy is not a new technique for pain control in upper limb CRPS. It is a predictable technique with accurate lesioning of the nerve (ganglion) and minimal non-intentional damage to surrounding structures [5]. However, there are still few reports on RF sympathectomy for upper limb CRPS. We report a case series of four patients with CRPS of the upper limb secondary to brachial plexus injury, all of whom failed conservative treatment and stellate ganglion blockade but responded to T2 and T3 sympathectomy.
After approval was granted by the Clinical Research Center and Ethics Committee, records of all patients with the diagnosis of CRPS were reviewed. A total of 5 patients were identified, but only 4 patients agreed to undergo T2 and T3 RF sympathectomy after receiving an explanation of the risks and benefits of the procedure. The 4 patients were all male and all presented with total unilateral upper limb paralysis. The brachial plexus injuries involved were, in all cases, the result of motor vehicle accidents. The diagnosis of CRPS was based on the International Association for the Study of Pain (IASP) diagnostic criteria [6]. Characteristics of the 4 patients are summarized in Table 1. During initial management, stellate ganglion blockades had been administered but were not found to provide satisfactory pain relief.
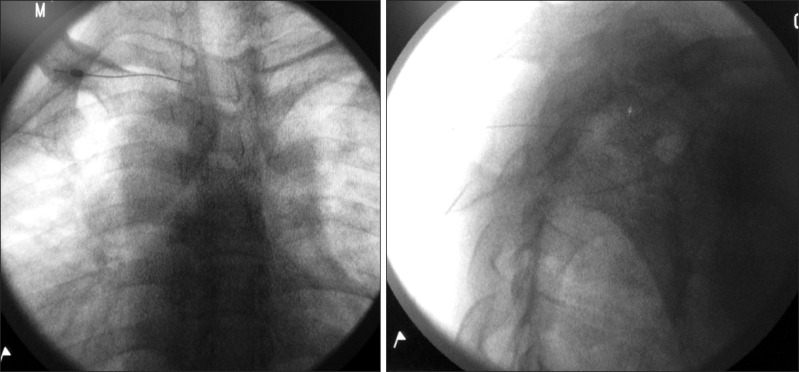
All patients were subsequently offered diagnostic T2 ganglion blockade, followed by RF sympathectomy of T2 and T3 ganglia. Both the diagnostic block and RF sympathectomy were performed with the patient in the prone position. The diagnostic block was performed at T2 level and an adequate volume of local anesthetic and dye was injected to ensure that spread of the local anesthetic reached T3 level. Under fluoroscopic guidance, the T2 vertebral body was identified in an anteroposterior view. In order to square the T2 vertebral body, the fluoroscope was obliqued at approximately 20° toward the ipsilateral side and was then rotated approximately 20° in a cephalad direction. The skin entry point was at the lateral edge of the T2 vertebral body just cephalad to the third rib. From surface anatomy, the skin entry point was kept within 4 cm from the spinous process to reduce the risk of pneumothorax. A 25 gauge, 10 cm Spinocan needle (B. Braun, Melsungen AG, Germany) was advanced toward the lateral border of the T2 vertebral body above the third rib in a tunnel view. With the aid of lateral, anteroposterior, and oblique fluoroscopic views, the needle was further advanced with the needle sliding along the lateral aspect of the vertebral body. The final location of the needle tip was at the posterior third of the vertebral body and midline in cephalocaudad relation in lateral view (Fig. 1). Once satisfactory placement of the needle tip was achieved, 0.5 to 1 ml of Ommipac 240 mg/ml was injected. The dye was observed to spread up and down the thoracic vertebral column, in both the anteroposterior and lateral views. A total of 5 ml of levobupivacaine 0.25% with triamcinolone acetate 40 mg was then injected. The diagnostic block was considered effective if a greater than 75% reduction in VAS (visual analogue scale) pain score for the whole upper limb was achieved for at least 6 hours.
For RF sympathectomy, a 22 gauge, 10 cm curved, sharp RF insulated needle with an active tip of 10 mm and Cosman G4 RF generator (Cosman Medical, Inc., Burlington, MA, USA) were used. Needle entry was performed and the final placement of the needle tip was located as per the technique described previously. Once the correct position was confirmed, a 10 cm CSK-TC10 electrode was introduced through the RF needle. Before lesioning, a sensory (at 50 Hz, up to 0.6 V) and motor (at 2 Hz, up to 1.2 V) test stimulation was performed to verify the location. If the patient experienced no dermatome-related sensation and had no intercostal muscle contractions, the needle positions were deemed satisfactory. Prior to lesioning, a total of levobupivacaine 0.25% 3 ml and triamcinolone acetate 40 mg were injected. Two lesions were made with the needle angled in the medial-cephalad and medial-caudad directions to increase the lesion area. The RF lesioning settings were set for 60 seconds at 80℃. The RF procedure was performed at both the T2 and T3 levels in this manner.
All patients experienced greater than 75% pain relief after diagnostic block at the T2 level and RF sympathectomy. At the 6-month follow-up, all patients were still experiencing more than 50% pain relief compared to their pain score prior to RF sympathectomy (Table 2). At the time the study was conducted, the mean duration of follow-up was 7.5 months. All of the study patients underwent only one RF sympathectomy, except for patient 1, who underwent another RF sympathectomy 9 months later. After the second RF sympathectomy, patient 1 experienced a pain relief effect similar to that of the previous procedure. All patients were able to decrease their oral analgesic dosage by more than 50% during the course of follow-up (Table 3). No complications attributed to thoracic RF sympathectomy were noted.
Go to : 
Neuropathic pain secondary to brachial plexus injury is one of the most severe types of pain experienced. It is commonly associated with the development of CRPS. This form of pain can be persistent and resistant to treatment [1]. The treatment for CRPS involves a multidisciplinary approach combining pain management, psychological treatment and support, and rehabilitation, in order to control pain and to restore limb function and prevent complications, e.g., contractures and osteoporosis [7]. Before CRPS begins to develop, early surgical repair of injured brachial plexus has shown promising results in both pain reduction and functional recovery [1]. This is also true for spinal cord stimulation, as various reports have shown favorable outcomes of spinal cord stimulation in the management of CRPS [8]. However, expertise in brachial plexus surgical repair and spinal cord stimulation is not widely available, as in our institution. Rehabilitation in patients with brachial plexus injury is often hampered by the development of CRPS. Early diagnosis and treatment of CRPS has been shown to result in better treatment outcomes [7]. While there is no curative treatment for CRPS, sympathetic blockade is found to be effective in controlling pain by decreasing abnormal hyperactive sympathetic tone.
A comprehensive understanding of the anatomy of the upper limb sympathetic system is crucial in the management of CRPS secondary to posttraumatic brachial plexus injury. With considerable anatomical variations in the sympathetic supply to the upper limb, and also with the existence of the nerve of Kuntz, conclusions concerning the efficacy of stellate ganglion blockade, especially as a long-term treatment for CRPS, could not be drawn until recently [3,9]. Sympathetic ganglion cell bodies that supply the upper limbs originate in the intermediolateral horn of the spinal cord from level T2 to T8. Preganglionic fibers travel to the sympathetic chain via white rami communicantes. Here, they ascend cephalad and synapse with postganglionic fibers, primarily in T2, but also in T3, in the stellate ganglia and in the middle cervical ganglia. By blocking both ganglia which are the crucial synaptic stations, the entire synaptic supply for the upper limbs can be blocked [10]. This could explain the reasonable efficacy of RF sympathectomy in controlling upper limb CRPS in the present study, even at 6 months post-sympathectomy. Besides CRPS, thoracic sympathectomy has been used for treatment of neuropathic pain in the thorax or upper abdominal viscera, pain related to herpes zoster or postherpetic neuralgia, and phantom breast pain after mastectomy. Other indications include vascular insufficiency leading to ischemia, Raynaud's disease, Buerger's disease, and frost injuries of the upper limbs [10]. Additional non-pain related conditions which are indications for thoracic sympathectomy include hyperhidrosis and facial flushing [11].
Despite a lengthy literature search, only a few studies were identified describing upper thoracic sympathectomy for severe CRPS. In one study, Agarwal-Kozlowski et al. demonstrated that significant pain reduction was achieved by using computed tomography-guided catheter injection of 80.75% ethanol [12]. The spread of ethanol might cover a wider area of neurolysis with a higher success rate, but the spread would not be predictable. Thus, unwanted destruction or irritation of surrounding structures might occur. In another study, in a series of twenty-four patients with sympathetically mediated pain, Herz et al. achieved a 71% overall success rate with surgical T2 sympathetic ganglionectomy. This technique, however, was invasive and subjected patients to surgical complications, which were mentioned in the study [13]. Thoracoscopic sympathectomy gained wide acceptance among surgeons and was claimed to be less invasive than the open technique [14]. Both open and endoscopic approaches require surgical expertise, which might not be available in every institution. In addition, both approaches pose a significant challenge to anesthetists and subject patients to surgical complications. As for the percutaneous technique, Wilkinson performed 148 RF sympathectomies in patients with various conditions, with symptomatic pneumothorax occurring in 6 patients [5].
The drawbacks of this procedure are the procedurerelated complications and the difficulty in achieving correct placement of the needle tip in a relatively crowded thoracic region. The most common complication related to thoracic sympathectomy is pneumothorax. This complication, however, could be minimized by keeping the needle entry point within 4 cm from the spinous process and advancing the needle with the "hugging the vertebral body" technique [10]. Besides pneumothorax, injury to the intercostal nerve is also a possible complication, if sensory and motor stimulations were not performed adequately prior to RF lesioning [5]. With further advancements in imaging techniques, e.g., CT scan [12] and fluoroscopy with cone-beam CT [15], more precise sympathectomies with fewer complications should be achievable in the near future. Successful sympathectomy is usually accompanied by temperature elevation of the ipsilateral limb [5]. As this report was not planned for research or publication purposes initially, no objective thermographic measurement of limb temperature was done, but all respondents reported experiencing noticeable elevation in limb temperature. Considerable anatomical variation in the sympathetic supply to the upper limb has been observed [4], which may explain the high incidence of recurrence of pain in the upper limb after thoracic sympathectomy. CRPS is a collection of various types of pain, with sympathetically mediated pain being the major component. Thus, although sympathectomy might result in a significant reduction in pain, it would not completely eradicate all of the pain, as observed in the present study.
This case series illustrates the success of percutaneous T2 and T3 RF sympathectomy via the multimodal approach to chronic pain management in controlling the pain suffered by patients with CRPS secondary to brachial plexus injury. When surgical repair of the brachial plexus and spinal cord stimulation are not available, percutaneous T2 and T3 RF sympathectomy could be considered in patients with type II CRPS.
Go to : 
References
1. Htut M, Misra P, Anand P, Birch R, Carlstedt T. Pain phenomena and sensory recovery following brachial plexus avulsion injury and surgical repairs. J Hand Surg Br. 2006; 31:596–605. PMID: 16822598.

2. Boas RA. Sympathetic nerve blocks: in search of a role. Reg Anesth Pain Med. 1998; 23:292–305. PMID: 9613543.
3. Yucel I, Demiraran Y, Ozturan K, Degirmenci E. Complex regional pain syndrome type I: efficacy of stellate ganglion blockade. J Orthop Traumatol. 2009; 10:179–183. PMID: 19888550.

4. Ramsaroop L, Partab P, Singh B, Satyapal KS. Thoracic origin of a sympathetic supply to the upper limb: the 'nerve of Kuntz' revisited. J Anat. 2001; 199:675–682. PMID: 11787821.

5. Wilkinson HA. Percutaneous radiofrequency upper thoracic sympathectomy. Neurosurgery. 1996; 38:715–725. PMID: 8692390.

6. Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle (WA): IASP Press;1994. p. 40–43.
7. Stanton-Hicks MD, Burton AW, Bruehl SP, Carr DB, Harden RN, Hassenbusch SJ, et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract. 2002; 2:1–16. PMID: 17134466.

8. Kim WY, Moon DE, Choi JH, Park CM, Han SM, Kim SH. The effect of spinal cord stimulation in patients with complex regional pain syndrome. Korean J Pain. 2006; 19:152–158.

9. McCormack AC, Jarral OA, Shipolini AR, McCormack DJ. Does the nerve of Kuntz exist? Interact Cardiovasc Thorac Surg. 2011; 13:175–178. PMID: 21602419.

10. Raj PP, Erdine S. Pain-relieving procedures: the illustrated guide. 1st ed. West Sussex: Wiley-Blackwell;2012. p. 244–249.
11. Yang JY, Kim C, Han KR, Cho HW, Kim EJ. Dorsal percutaneous thoracic sympathetic ganglion block with alcohol for the treatment of palmar hyperhidrosis. Korean J Pain. 2005; 18:171–175.

12. Agarwal-Kozlowski K, Lorke DE, Habermann CR, Schulte am, Beck H. Interventional management of intractable sympathetically mediated pain by computed tomography-guided catheter implantation for block and neuroablation of the thoracic sympathetic chain: technical approach and review of 322 procedures. Anaesthesia. 2011; 66:699–708. PMID: 21564048.

13. Herz DA, Looman JE, Ford RD, Gostine ML, Davis FN, VandenBerg WC. Second thoracic sympathetic ganglionectomy in sympathetically maintained pain. J Pain Symptom Manage. 1993; 8:483–491. PMID: 7525779.

14. Rizzo M, Balderson SS, Harpole DH, Levin LS. Thoracoscopic sympathectomy in the management of vasomotor disturbances and complex regional painsyndrome of the hand. Orthopedics. 2004; 27:49–52. PMID: 14763530.

15. Hirose M, Tabata M, Sakai M, Takeuchi K. C-arm fluoroscopic cone-beam CT for guidance of chemical thoracic sympathectomy. J Anesth. 2011; 25:142–143. PMID: 21116658.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download