Abstract
Background
Ketamine, an N-methyl-D-aspartate receptor antagonist, might play a role in postoperative analgesia, but its effect on postoperative pain after caesarean section varies with study design. We investigated whether the preemptive administration of low-dose intravenous ketamine decreases postoperative opioid requirement and postoperative pain in parturients receiving intravenous fentanyl with patient-controlled analgesia (PCA) following caesarean section.
Methods
Spinal anesthesia was performed in 40 parturients scheduled for elective caesarean section. Patients in the ketamine group received a 0.5 mg/kg ketamine bolus intravenously followed by 0.25 mg/kg/h continuous infusion during the operation. The control group received the same volume of normal saline. Immediately after surgery, the patients were connected to a PCA device set to deliver 25-µg fentanyl as an intravenous bolus with a 15-min lockout interval and no continuous dose. Postoperative pain was assessed using the cumulative dose of fentanyl and visual analog scale (VAS) scores at 2, 6, 24, and 48 h postoperatively.
Go to : 
The greatest concern of mothers undergoing cesarean section is postoperative pain [1]. When pain is not controlled appropriately, it can interrupt contact between mother and newborn and when postoperative pain is severe, it can delay early ambulation and discharge; thus, appropriate pain control after cesarean section is essential.
Pain management after cesarean section can be accomplished with various methods, but generally it is achieved through a patient-controlled analgesia (PCA) device with opioids. Various drugs, such as non-steroidal anti-inflammatory drugs, local anesthetics, α2-adrenaline receptor antagonists, and ketamine, have been used to enhance the effect of PCA and reduce complications that can occur when large dose opioids are used alone [2-4]. Among these, the frequency of using low dose ketamine as an adjuvant drug for local anesthesia or opioids in multimodal pain therapy is increasing gradually [5].
Ketamine is a non-competitive antagonist of the Nmethyl-D-aspartate receptor (NMDA-R) that inhibits central sensitization and has a preemptive analgesic effect to relieve postoperative pain [6,7]. In particular, even when ketamine is administered in low doses with no anesthesia effect, it suppresses facilitation of pain related to NMDA-R [8].
Reports regarding the preemptive analgesic effect of ketamine administered to patients undergoing cesarean section are inconsistent [9-13]. Ketamine administered during surgery reportedly has a preemptive analgesic effect, particularly in mothers receiving cesarean section under spinal anesthesia [10,11], whereas other reports contradict this result [13]. The contrasting results are considered to be due to differences in administration time and dosages of ketamine, anesthesia method, and in the method for controlling postoperative pain.
The purpose of this study was to investigate the preemptive analgesic effect of low-dose ketamine in pregnant mothers undergoing cesarean section under spinal anesthesia and receiving opioids through an intravenous PCA device after the operation.
Go to : 
The study was conducted on 40 pregnant mothers of ASA class 1-2, between 37-42 weeks of pregnancy, who were scheduled for cesarean section under spinal anesthesia. This study was approved by the Institutional Review Board, and consent was obtained from all subjects. Patients with psychological diseases, difficulties communicating, allergies to local anesthesia, inflammation in the spinal puncture area, coagulation disorder, administered analgesics, and those who underwent an emergency operation were excluded. The subjects were educated about PCA and the visual analogue scale (VAS, 0 = no pain, 10 = worst pain imaginable) before the operation.
No premedication was administered, and patients were monitored by electrocardiogram, non-invasive arterial blood pressure, and pulse oximetry when they entered the operating room. In the left lateral decubitus position, the dura was punctured between the L3-4 intervertebral space using a 24-gauge Quincke spinal needle. After checking for cerebrospinal fluid, 10 mg 0.5% hyperbaric bupivacaine (Marcaine Spinal Heavy, AstraZeneca, UK) was injected. When systolic blood pressure decreased to < 90 mmHg, or 30% of the pre-anesthetic blood pressure, it was corrected by administering 5 mg ephedrine or 50 µg phenylephrine. After administering bupivacaine, the height of the sensory block was checked by pinprick at 5-min intervals, and the maximum sensory block height was recorded. Fentanyl 50 µg was administered up to two times based on either the patient's request or the decision of the anesthesiologist during surgery; if pain was still not controlled, the method was changed to general anesthesia, and the case was not included in the study analysis. The occurrence of over-sedation, nightmares, and hallucinations was examined as adverse side effects of ketamine administered during surgery. Over-sedation was defined when the patient could not remember the moment of childbirth.
Patients were assigned to a control or ketamine group through random allocation using a computer. After spinal anesthesia and before making the incision, the ketamine group was intravenously (IV) injected with 0.5 mg/kg ketamine, and ketamine was continually infused at 0.25 mg/kg/h until the end of the operation [14]. The control group was IV injected with the same volume of normal saline. The ketamine and saline solution used in the experiment were administered using the double-blind method.
After suturing the skin, a PCA device (AutoMed 3400, Acemedical, Seoul, Korea) was connected to the IV route of the patient. Fentanyl (25 µg/ml) was prepared for the PCA, and both groups were set with a demand dose of 1 ml and a lockout interval of 15 min without a continuous dose [15].
The cumulative dose of fentanyl administered through the PCA device at 2, 6, 24, and 48 h after surgery was measured as the primary outcome of this study. The resting and coughing VAS scores for the same time period were measured as secondary outcomes. Patient satisfaction (4 = very satisfied, 3 = satisfied, 2 = average, 1 = unsatisfied, 0 = very unsatisfied) regarding pain control was measured 48 h after the operation. When the resting VAS score was ≥ 5 or when the patient requested, ketorolac 30 mg were additionally injected intramuscularly (IM), and the number of administered patients was recorded. All values were measured by an anesthesiologist without knowledge of the patient grouping.
The cumulative fentanyl dose is expressed as mean ± standard deviation and was compared using the unpaired t-test. The VAS scores and patient satisfaction are expressed as median values and quartiles and compared using the Mann-Whitney U-test. The number of patients administered fentanyl during surgery and the number of patients administered ketorolac after surgery were compared with Fisher's exact test. A P value < 0.05 was considered to indicate significance. SPSS for Windows version 11.0 was used for the statistical analysis. In a pilot study, a 30% decrease from 1,000 µg in the mean cumulative dose of fentanyl during 48 h postoperatively was presumed to be a significant decrease, the required sample size was 16 for each group (α = 0.05, β = 0.2, standard deviation = 300 µg), and 20 subjects were assigned to each group considering a drop-out rate of 20%.
Go to : 
One subject in the ketamine group and three in the control group were excluded due to a failed spinal puncture or a switch to general anesthesia. Thus, the statistical analysis was performed on the values measured from the remaining 19 patients in the ketamine group and 17 in the control group.
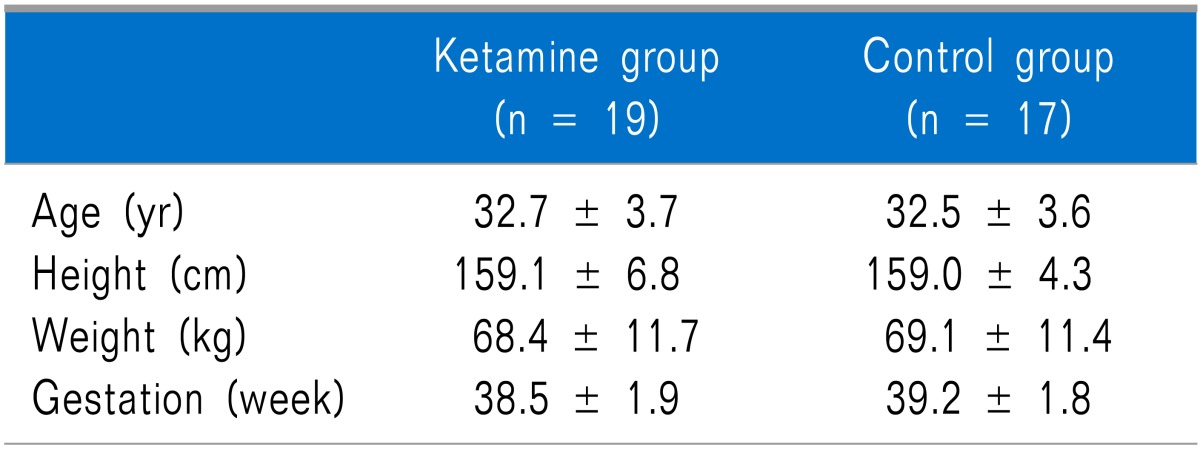
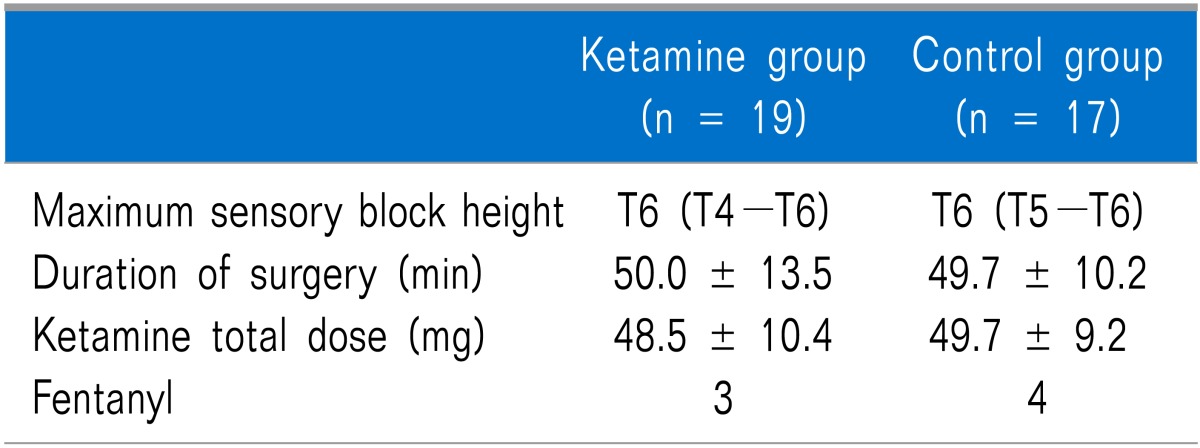
No differences in age, height, weight, or length of pregnancy were observed between the groups (Table 1). No differences in the maximum height of the sensory block, operating time, total administered dose of ketamine during surgery, and number of patients administered fentanyl during the operation were observed between the groups (Table 2).
Three subjects in the ketamine group did not remember the moment of childbirth due to over-sedation, but the difference was not significant. Nightmares and hallucinations did not occur in either group.
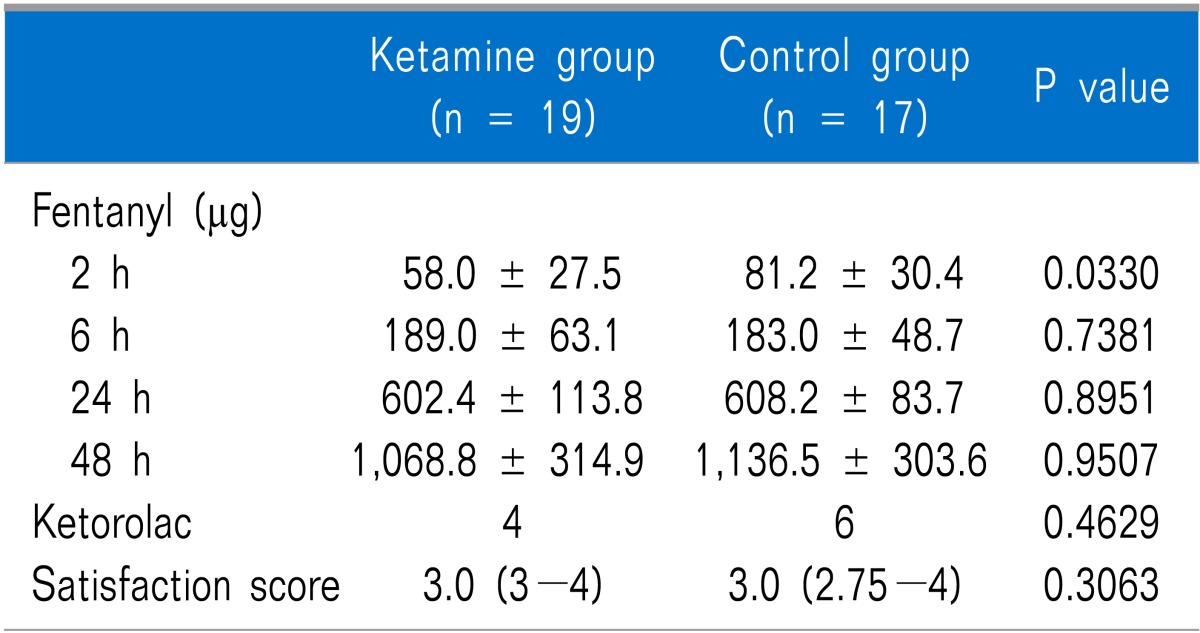
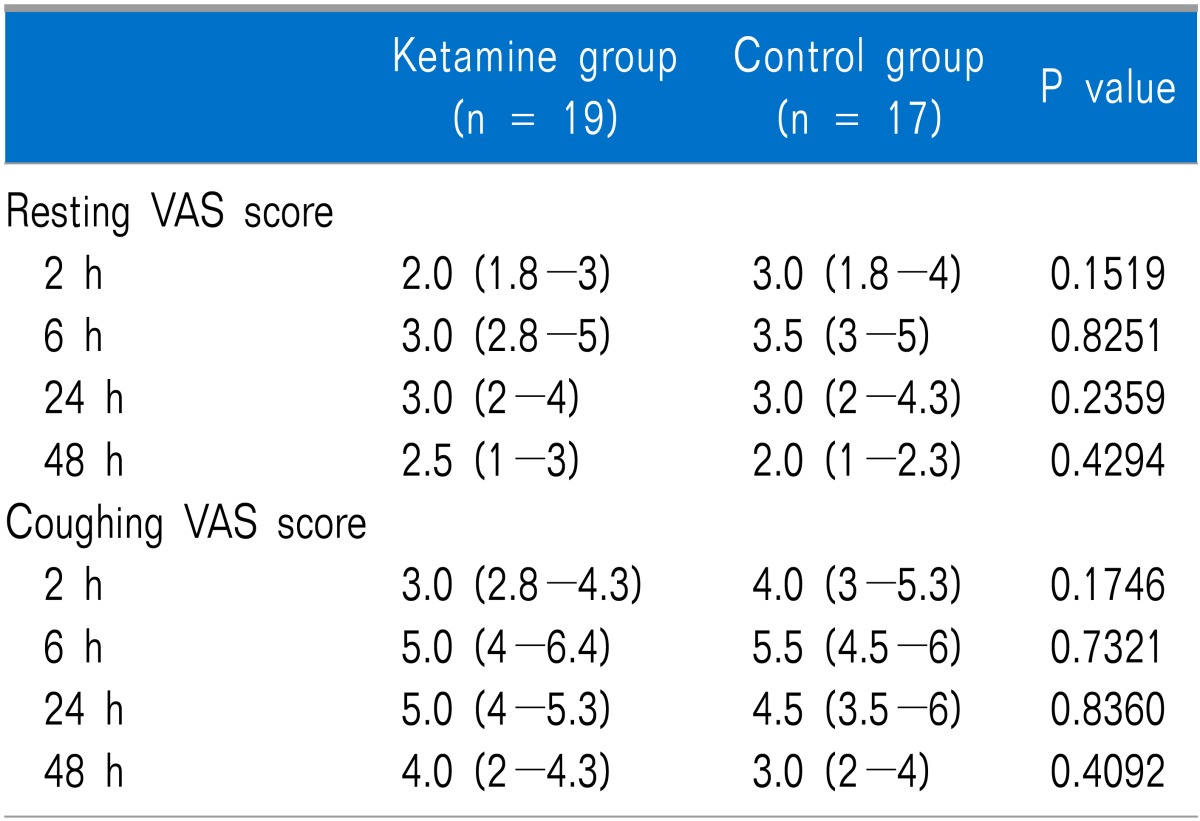
The cumulative dose of fentanyl measured at 2 h after surgery was significantly lower in the ketamine group than that in the control group (P = 0.033), but no significant difference was observed at 6, 24, or 48 h after surgery (Table 3). No differences in the number of patients administered ketorolac up to 48 h after surgery or in patient satisfaction were observed between the groups. The resting and coughing VAS scores measured at 2, 6, 24, and 48 h after surgery were not significantly different between the two groups (Table 4).
Go to : 
When ketamine was administered to pregnant mothers receiving a cesarean section under spinal anesthesia from before the skin incision to the end of the surgery, the cumulative dose of fentanyl was lower than that in the control group at 2 h after surgery, but no difference was observed between the groups at 6, 24, or 48 h after surgery. Furthermore, no differences in VAS score, number of patients administered ketorolac, or patient satisfaction were observed between the two groups.
In studies of the preemptive analgesic effect of ketamine, the pain reduction effect started immediately after the operation and continued up to 24 or 48 h after surgery [9-11]. In the present study, the cumulative dose of fentanyl 2 h after surgery in the ketamine group was lower than that of the control group, but it was difficult to determine whether this result was due by the preemptive analgesic effect of ketamine. Ketamine has an analgesic effect at plasma concentrations of ≥ 100 ng/ml [16], and the plasma concentration is maintained at this level or higher up to 1-2 h after the operation when administered at a dose of 1 mg/kg [9]. We did not measure the plasma concentration of ketamine, but an initial dose of 0.5 mg/kg and an additional continuous dose of 0.25 mg/kg/h were injected until the end of surgery; thus, the plasma concentration of ketamine was probably high enough to have a direct analgesic effect at 2 h after surgery. No differences was observed in the cumulative dose of fentanyl beginning 6 h after surgeryand in VAS scores during the experiment; therefore, the ketamine administered in this study was considered to have no preemptive analgesic effect. This is contrary to reports that ketamine administered to pregnant mothers undergoing cesarean section reduces postoperative pain or opioid requirements [9-11], but similar to other studies which reported no such effect [12,13].
The administered dose of ketamine may explain the preemptive analgesic effect of ketamine. Low-dose ketamine is defined as an IV injection or epidural administration of ≤ 1.0 mg/kg, or an IM injection of ≤ 2.0 mg/kg [17]. Reza et al. [12] reported that the postoperative morphine requirement was not reduced in their study when 0.5 mg/kg ketamine was administered, but the postoperative analgesic requirement was reduced when 1.0 mg/kg was administered in a study by Ngan Kee et al. [9]. They explained that this was because when a high dose of ketamine is administered, it can reach a relatively higher plasma concentration to suppress NMDA-R activation compared to that of low-dose administration. However, there are other reports in which 0.5 mg/kg of ketamine was helpful for relieving postoperative pain after abdominal surgery [18,19], and even that the analgesic requirement after cesarean section was reduced with administration of a low dose of 0.15 mg/kg [10,11]. There is also a report that the morphine requirement was not different in three groups of cesarean section patients administered 0.25, 0.5, or 1.0 mg/kg of ketamine [20]; thus, it is possible that the preemptive analgesic effect of ketamine is not dose dependent. Hence, an insufficient dose is unlikely to be the cause of the lack of relief of postoperative pain in our study. Moreover, a high dose of ketamine can cause psychomimetic effects and the mother may not remember the moment of childbirth; therefore, it is not an appropriate choice for mothers receiving spinal anesthesia.
Researches into the preemptive analgesic effect of ketamine have been conducted mainly in patients undergoing general anesthesia [17,21]. General anesthesia using inhalation or intravenous anesthetics does not completely block sensory transmission regarding nociceptive input; therefore, secondary hyperalgesia can occur due to activation of NMDA-R causing central sensitization and wind-up. This can be prevented by administering an NMDA-R antagonist. When sensory transmission is completely blocked by performing regional anesthesia using a local anesthetic, NMDA-R is not activated, so there is no additional effect of an NMDA-R antagonist other than the preemptive analgesic effect of the local anesthetic. However, this is open to dispute because there are studies [10,11] reporting the preemptive analgesic effect of ketamine in patients undergoing spinal anesthesia using bupivacaine. In those studies, the pain of the patients during surgery was not addressed; thus, there is the possibility that sufficient height of the sensory block was not achieved. In our study, considering that the number of patients administered fentanyl due to intraoperative pain was relatively small (ketamine group: three, control group: four), sufficient height of the sensory block was considered one of the reasons for the lack of a preemptive analgesic effect of ketamine, but further research regarding this point is necessary.
The preemptive effect of ketamine can be determined by the degree of postoperative pain. The degree of postoperative pain after cesarean section can differ according to analgesic method. When several analgesic drugs are used to sufficiently control pain and are administered appropriately, the preemptive analgesic effect of ketamine may be hidden, and when only one type of drug is administered after surgery, there is a high possibility of insufficient pain control and the effect of ketamine could be larger [13]. In our study, pain was controlled with PCA using fentanyl and ketorolac was additionally administered as needed; thus, a relatively sufficient amount of analgesic drug may be the reason for the lack of an effect of ketamine. However, this is open to dispute because ketamine administered before the incision was effective in mothers who received morphine pain control with a PCA after cesarean section [9].
The effect of ketamine on postoperative pain appears differently according to the type and extensiveness of the operation, and the preemptive analgesic effect of ketamine is greater in cases that the postoperative pain is more severe. In a systematic review by Laskowski et al. [22], the effect of ketamine was greater in upper abdominal surgery, thoracotomy, or when the VAS score was ≥ 7, compared to that of lower abdominal surgery or when the VAS score was < 4. The postoperative pain after cesarean section was not strong enough for the effect of ketamine to be clearly determined, which could be why the resting and coughing VAS scores were not different between the two groups in our study. Different results may have been observed if a moving VAS score was measured, where the degree of pain is relatively stronger. However, not only is this impossible to measure 2 h after surgery, it would also have been difficult to obtain the cooperation of the subjects later; thus, it was not selected as an outcome measurement.
Suppa et al. [23] reported that the postoperative morphine requirement is reduced when 0.5 mg/kg S-ketamine IV is injected 10 min after childbirth and administered at a speed of 2 µg/kg/h for 12 h in mothers receiving cesarean section under spinal anesthesia. They reported that preemptive analgesia is insufficient to prevent sensitization, and that preventive treatment should be performed during the entire perioperative period. However, there are also claims that the effect of ketamine on postoperative pain is unrelated to administration time or period [22], so there is a need for further research regarding this area.
Different results have been reported on the psychomimetic side effects of low dose ketamine administered to mothers undergoing cesarean section according to the research method. Menkiti et al. [11] reported that the difference in frequency of adverse side effects such as sedation, headache, or hallucinations was not significant between a ketamine and control group, but the result was not reliable due to the small number of subjects. Suppa et al. [23] reported no severe complications in a group administered ketamine, but significant increases in drowsiness, dizziness, and nystagmus were observed. In our study, severe complications were not significantly different between the groups, but three patients did not remember the moment of childbirth. Considering the specificity of pregnant mothers, a randomized controlled study of the psychomimetic side effects of ketamine as the main research goal is necessary.
A limitation of this study was that the effect of ketamine administered before childbirth on the fetus was not measured. However, we used a relatively small amount of ketamine, and, according to other studies [20,24], the effect of ketamine administered for anesthetic induction or controlling delivery pain during cesarean sections on the fetus is not clinically important. Ketamine is neurotoxic to developing brains, but this is an adverse side effect appearing in animal experimentation in which high doses were administered for long periods of time, and there is no confirmation of this effect on the human body [25].
In conclusion, 0.5 mg/kg ketamine was injected IV before the skin incision and infused continually at 0.25 mg/kg/h until the end of the operation in pregnant mothers receiving cesarean section under spinal anesthesia, but this treatment did not reduce the fentanyl requirement or postoperative VAS score. Further research is needed regarding the postoperative pain relief effect and complications according to administration time, period, and dosage of ketamine in pregnant mothers receiving cesarean section under spinal anesthesia.
Go to : 
ACKNOWLEDGEMENTS
This work was supported in part by the Soonchunhyang University Research Fund.
Go to : 
References
1. Carvalho B, Cohen SE, Lipman SS, Fuller A, Mathusamy AD, Macario A. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg. 2005; 101:1182–1187. PMID: 16192541.

2. Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004; 98:153–158. PMID: 14693611.

3. Cassuto J, Wallin G, Högström S, Faxén A, Rimbäck G. Inhibition of postoperative pain by continuous low-dose intravenous infusion of lidocaine. Anesth Analg. 1985; 64:971–974. PMID: 3898920.

4. El-Tahan MR, Warda OM, Yasseen AM, Attallah MM, Matter MK. A randomized study of the effects of preoperative ketorolac on general anaesthesia for caesarean section. Int J Obstet Anesth. 2007; 16:214–220. PMID: 17459695.

5. Weinbroum AA. Non-opioid IV adjuvants in the perioperative period: pharmacological and clinical aspects of ketamine and gabapentinoids. Pharmacol Res. 2012; 65:411–429. PMID: 22311381.

6. Yamamura T, Harada K, Okamura A, Kemmotsu O. Is the site of action of ketamine anesthesia the N-methyl-D-aspartate receptor. Anesthesiology. 1990; 72:704–710. PMID: 1969718.

7. Guirimand F, Dupont X, Brasseur L, Chauvin M, Bouhassira D. The effects of ketamine on the temporal summation (wind-up) of the R(III) nociceptive flexion reflex and pain in humans. Anesth Analg. 2000; 90:408–414. PMID: 10648330.

8. Aida S, Yamakura T, Baba H, Taga K, Fukuda S, Shimoji K. Preemptive analgesia by intravenous low-dose ketamine and epidural morphine in gastrectomy: a randomized double-blind study. Anesthesiology. 2000; 92:1624–1630. PMID: 10839912.

9. Ngan Kee WD, Khaw KS, Ma ML, Mainland PA, Gin T. Postoperative analgesic requirement after cesarean section: a comparison of anesthetic induction with ketamine or thiopental. Anesth Analg. 1997; 85:1294–1298. PMID: 9390597.
10. Sen S, Ozmert G, Aydin ON, Baran N, Caliskan E. The persisting analgesic effect of low-dose intravenous ketamine after spinal anaesthesia for caesarean section. Eur J Anaesthesiol. 2005; 22:518–523. PMID: 16045141.

11. Menkiti ID, Desalu I, Kushimo OT. Low-dose intravenous ketamine improves postoperative analgesia after caesarean delivery with spinal bupivacaine in African parturients. Int J Obstet Anesth. 2012; 21:217–221. PMID: 22658477.

12. Reza FM, Zahra F, Esmaeel F, Hossein A. Preemptive analgesic effect of ketamine in patients undergoing elective cesarean section. Clin J Pain. 2010; 26:223–226. PMID: 20173436.

13. Bauchat JR, Higgins N, Wojciechowski KG, McCarthy RJ, Toledo P, Wong CA. Low-dose ketamine with multimodal postcesarean delivery analgesia: a randomized controlled trial. Int J Obstet Anesth. 2011; 20:3–9. PMID: 21224020.

14. De Kock M, Lavand'homme P, Waterloos H. 'Balanced analgesia' in the perioperative period: is there a place for ketamine? Pain. 2001; 92:373–380. PMID: 11376910.

15. Howell PR, Gambling DR, Pavy T, McMorland G, Douglas MJ. Patient-controlled analgesia following caesarean section under general anaesthesia: a comparison of fentanyl with morphine. Can J Anaesth. 1995; 42:41–45. PMID: 7889583.

16. Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981; 53:27–30. PMID: 7459184.

17. Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999; 82:111–125. PMID: 10467917.

18. Behdad A, Hosseinpour M, Khorasani P. Preemptive use of ketamine on post operative pain of appendectomy. Korean J Pain. 2011; 24:137–140. PMID: 21935491.

19. Fu ES, Miguel R, Scharf JE. Preemptive ketamine decreases postoperative narcotic requirements in patients undergoing abdominal surgery. Anesth Analg. 1997; 84:1086–1090. PMID: 9141936.

20. Bilgen S, Köner O, Türe H, Menda F, Fiçicioğlu C, Aykaç B. Effect of three different doses of ketamine prior to general anaesthesia on postoperative pain following Caesarean delivery: a prospective randomized study. Minerva Anestesiol. 2012; 78:442–449. PMID: 22240615.
21. Roytblat L, Korotkoruchko A, Katz J, Glazer M, Greemberg L, Fisher A. Postoperative pain: the effect of low-dose ketamine in addition to general anesthesia. Anesth Analg. 1993; 77:1161–1165. PMID: 8250308.
22. Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011; 58:911–923. PMID: 21773855.

23. Suppa E, Valente A, Catarci S, Zanfini BA, Draisci G. A study of low-dose S-ketamine infusion as "preventive" pain treatment for cesarean section with spinal anesthesia: benefits and side effects. Minerva Anestesiol. 2012; 78:774–781. PMID: 22374377.
24. Dich-Nielsen J, Holasek J. Ketamine as induction agent for caesarean section. Acta Anaesthesiol Scand. 1982; 26:139–142. PMID: 7102236.

25. Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007; 104:509–520. PMID: 17312200.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download