Abstract
Boney metastasis may lead to terrible suffering from debilitating pain. The most likely malignancies that spread to bone are prostate, breast, and lung. Painful osseous metastases are typically associated with multiple episodes of breakthrough pain which may occur with activities of daily living, weight bearing, lifting, coughing, and sneezing. Almost half of these breakthrough pain episodes are rapid in onset and short in duration and 44% of episodes are unpredictable. Treatment strategies include: analgesic approaches with "triple opioid therapy", bisphosphonates, chemotherapeutic agents, hormonal therapy, interventional and surgical approaches, steroids, radiation (external beam radiation, radiopharmaceuticals), ablative techniques (radiofrequency ablation, cryoablation), and intrathecal analgesics.
In 2008 there were over 12 million new cases of cancer diagnosed and 7.6 million deaths from cancer with up to 75-90% of patients with metastatic or advanced stage cancer experiencing significant cancer-induced pain [1-5].
Approximately half or more of patients diagnosed with cancer may experience bone pain. Bone is the third most common site of metastatic disease. Breast, lung, and prostate cancers are collectively responsible for about 80 percent of secondary metastatic bone disease. Other common types of cancer, such as thyroid, lung, and renal cell carcinomas, also display significant osteotropism. Cacrinomas are more likely to metastasize to bone than sarcomas. The axial skeleton is seeded more than the appendicular skeleton, particularly due to the persistence of red bone marrow in the former. The ribs, pelvis and spine are generally involved early with distal bone involvement being infrequent. Batson's vertebral venous plexus permits malignant cells to enter the vertebral circulation without first passing through the lungs. Malignant cell survival with the development of spinal metastases may occur is common due to the sluggish blood flow in this plexus. In general, when a tumor grows in bone it may become more of a challenge to achieve a "cure" status, and it may cause devastating clinical complications, such as intractable severe pain, pathological fractures, spinal cord and nerve compression, hypercalcemia, and bone marrow aplasia, collectively referred as "skeletal-related events". Not all patients with bone metastases have pain, but about 83% of patients with osseous metastases complain of pain at some point with wide variation in pattern and severity [6] (Fig. 1).
Cancer Induced Bone Pain (CIBP) often results in hospice or hospital admission and is associated with reduced quality of life, increased psychological distress and decreased physical and social functioning. With higher levels of disability, advanced illness and pain, comes an increased incidence of depression and anxiety [7].
CIBP does not exist as a single entity, but instead may be considered as a combination of background pain and breakthrough pain. Breakthrough pain (BTP) has been defined as 'a transitory exacerbation of pain experienced by the patient who has relatively stable and adequately controlled baseline pain'. Breakthrough pain can be divided into spontaneous pain at rest and incident pain (either volitional or non-volitional). Breakthrough pain was present in 75% of cases of CIBP [6]. Patients with breakthrough pain had greater interference on aspects of life (mood, relationships, sleep, activity, walking ability, work, enjoyment of life) than those with no breakthrough pain (P < 0.01). Almost half of breakthrough p ain episodes were rapid in onset (< 5 min) and short in duration (< 15 min). Forty-four per cent of patients with breakthrough pain had pain that was unpredictable [6]. These clinical characteristics make the successful treatment of CIBP challenging. This has been supported by other studies that have shown that up to 45% of patients with CIBP report poor pain control [8].
Treatment strategies have employed various therapies for the treatment of painful osseous metastases including: bisphosphonates, chemotherapeutic agents---mitoxantrone (a chemotherapeutic agent that inhibits DNA synthesis), hormonal therapy, interventional, and surgical approaches. Additional agents may include systemic analgesics, steroids, radiation, (external beam radiation, radiopharmaceuticals), and ablation (radiofrequency ablation (RFA) and cryoablation), and intrathecal analgesics.
In order for cancer cells to metastasize to bone and cause pain at least six things generally need to occur, including.
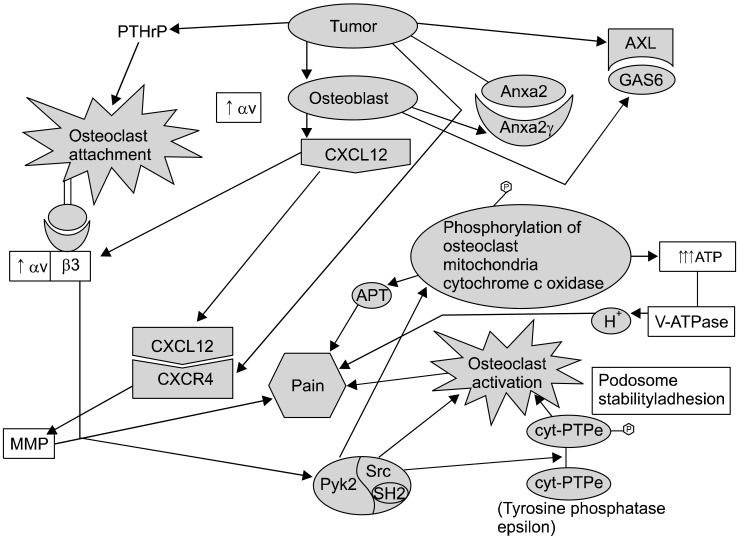
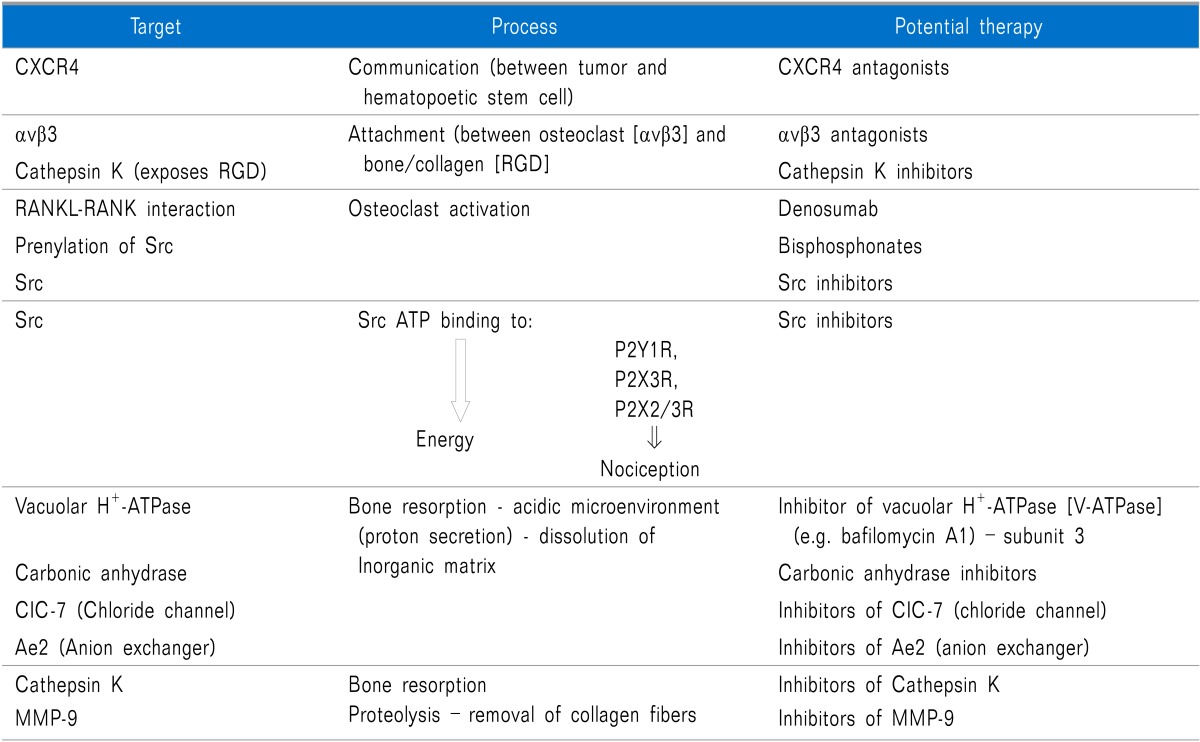
There needs to be interaction between the tumor cells and the bone marrow hematopoietic stem cells and the most important factor facilitating this interaction is CXCR4 signaling [stromal-d factor 1 (SDF-1; also referred to as CXCL12) binding to CXCR4].
The attachment/of osteoclasts to bone/collagen is largely due to the integrin αvβ3-facilitated by cathespin K exposing the RGD (Arg-Gly-Asp) sequence from collagen to αvβ3 (also known as the vitronectin receptors).
Osteoclast activation appears to contribute to painful osteolytic lytic/erosions. The interaction of RANKL and RANK as well as promotes osteoclast activation and interference with these interactions will lead to inhibition of osteoclast activation and pain. C-Src kinase activity is increased in response to integrin binding as well as RANKL/RANK interaction, and increased c-Src is involved in promoting osteoclast function/activation.
The resorption of bone may be considered as essentially two events; resorption of the organic matrix and resorption of the inorganic matrix.
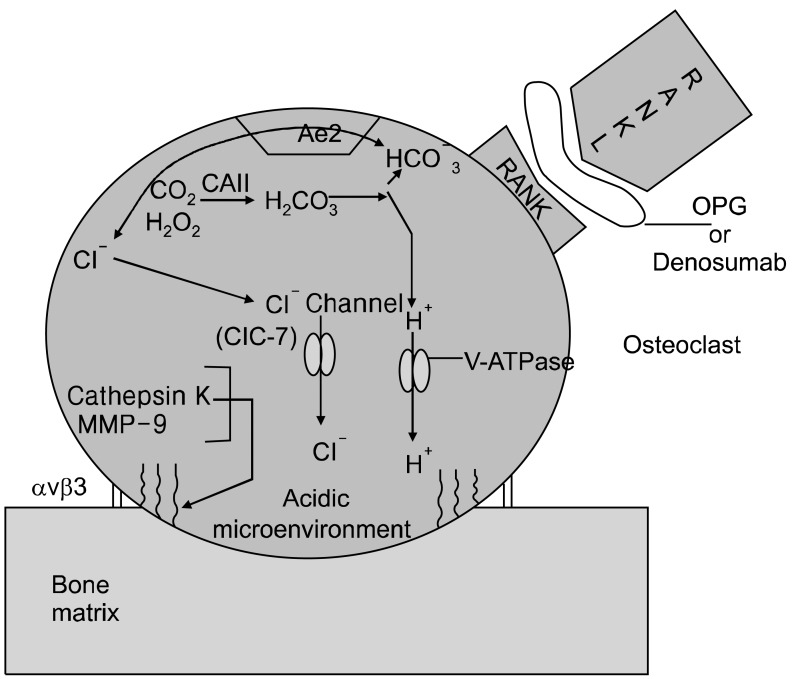
Cleavage of the type I collagen fibers is mainly mediated by the cysteine proteinase cathepsin K, which is active at low pH [9], and performs almost complete removal of the type I collagen fibers [10]. The MMPs are also involved in the degradation of the organic matrix of the bones; however, their precise role is remains uncertain (Fig. 2).
The resorption of the inorganic matrix of bone requires two major factors: energy (ATP) and acid (HCI). The osteoclasts generate H+ and Cl- utilizing carbonic anhydrase II that catalyzes conversion of carbon dioxide and water into carbonic acid, which in turn dissociates into hydrogen ion (H+) and bicarbonate (HCO3-) [11]. The HCO3- ions are then exchanged for Cl- through the basolaterally located Anion Exchanger 2 (AE2) [12], providing the Cl- ions required for acidification [HCl] occurring in the resorption lacuna (Fig. 2).
Inside the sealing zone, bone resorption is induced by active secretion of protons to the bone surface through a specialized vacuolar type ATPase (V-ATPase) requiring ATP, containing the a3 subunit [13] and passive transport of chloride through the chloride channel [ClC-7], also to the bone surface [14] (Fig. 2). Hydrochloric acid lowers the pH to approximately 4.5, leading to dissolution of the inorganic matrix of bone [15].
Thus, involvement of vacuolar H+-ATPase and carbonic anhydrase are crucial to "digesting" bone with subsequent creation of osteolytic lesions. c-Src may contribute to bone resorption, in part by: 1) preventing the inhibitory effects of calcitonin on osteoclast function and facilitating osteoclast activation, 2) enhancing the normal organization of the osteoclast actin cytoskeleton and contributing to the formation of the "ruffled border" [after c-Src is recruited to the plasma membrane, 3) facilitating podosome activities by promoting a shift from stable focal adhesions with actin stress fibers to more dynamic podosome assemblies, 4) by phosphorylating cytochrome c oxidase within the mitochondria, thereby increasing cytochrome c oxidase activity, and subsequently contributing to the generation of high levels of ATP required for bone resorbing actions of osteoclasts [16] (Fig. 3). The ATP produced by c-Src-induced cytochrome c oxidase activity may be utilized by V-ATPase to provide energy for the proton pump to secrete hydrogen ions by the bone surface. Furthermore, the ATP generated may also contribute to nociception via binding to purinergic receptors (P2X2/3 and P2X3). Targeting major processes involved in painful osseous metastases may lead to novel potential future therapeutic agents (Table 1).
Treatment strategies for painful osseous metastases include multiple nonpharmacologic approaches. These may include: physical medicine approaches, tai chi, yoga, stretching modalities, heat, cold, galvanic ultrasound, behavioral medicine approaches, cognitive behavioral therapy, mediation, hypnosis, relaxation techniques, guided imagery, and acupuncture. Bradt and colleagues performed a systematic review indicating that music interventions may have beneficial effects on anxiety, pain, mood, and quality of life, in people with cancer [17]. Jane and colleagues conducted a randomized clinical trial was to compare the efficacy of message therapy (MT) to a social attention control condition on pain intensity, mood status, muscle relaxation, and sleep quality in a sample (n = 72) of Taiwanese cancer patients with bone metastases [18]. MT was shown to have beneficial within- or between-subjects effects on pain, mood, muscle relaxation, and sleep quality.
The "standard" or "traditional" pharmacologic approach to the treatment or palliation of painful osseous metastases follows the World Health Organization (WHO) analgesic stepladder guidelines approach to pain relief. An international WHO Expert Committee on cancer pain, chaired by Dr. Kathleen Foley of Memorial Sloan-Kettering Cancer Center, was convened in 1982, and in 1986 the WHO monograph Cancer Pain Relief was published [19]. By 1993 it has been translated into 22 languages [19]. The WHO guidelines have been prospectively and cross-culturally validated and shown to work well clinically [19]. Zech et al. published the largest prospective trial of WHO guidelines to date and achieved favorable pain control in 76% of 2118 cancer patients who were treated over a 10-year interval [20]. Analgesic agents which may play a role in the WHO guidelines approach include: non-opioid analgesics (acetaminophen, traditional or nonselective non-steroidal anti-inflammatory drugs [NSAIDs], cyclooxygenase-2 inhibitors), adjuvants (antidepressants, anticonvulsants, muscle relaxants, alpha-2 adrenergic agonists, n-methyl-d-aspartate [NMDA] receptor antagonists), and opioids/opioid-like analgesic agents.
Although the World Health Organization has recommended a three-step analgesic ladder for the treatment of cancer pain, it has been reported that 45% of patients with bone cancer pain (BCP) have inadequate and undermanaged pain control because of the relative ineffectiveness and adverse side effects of current pharmacotherapy [21].
NSAIDs appear to be particularly useful in patients with bone pain or pain related to inflammatory conditions and less useful in patients with neuropathic pain [22]. However, although clinicians regard anti-inflammatory agents as important drugs for the treatment of CIBP, this has largely been based on experience rather than a strong evidence base; and the use of traditional [nonselective (NS)] NSAIDs in CIBP has been questioned due to the lack of robust, clinical evidence [23].
Eisenberg and colleagues performed a meta-analysis of the published randomized controlled trials to assess the efficacy and safety of nonsteroidal antiinflammatory drugs (NSAIDs) in the treatment of cancer pain by meta-analyses of the published randomized control trials (RCTs) [24]. Twenty-five studies met inclusion criteria for analysis. Of these, 13 tested a single-dose effect, nine multiple-dose effects, and three both single- and multiple-dose effects of 16 different NSAIDs in a total of 1,545 patients [24]. Better pain relief was obtained from NSAIDs than placebo in three scores summed pain intensity difference [SPID], peak pain relief, and total pain relief based on five or six studies, and in another score peak pain intensity difference [PPID] based on eight studies. Single doses of placebo produced a 15% to 36% rate of analgesia, whereas NSAIDs resulted roughly twice as much analgesia (31% to 60%). All differences between NSAIDs and placebo comparisons were statistically significant [24]. Pain was related to bone metastases in seven studies. Four studies enrolled patients with either malignant bone pain, non-bone malignant pain, or both, but results were not reported separately for bone-related and non-bone-related pain in any study. Three studies [25-27] examined the analgesic efficacy of NSAIDs specifically for malignant bone pain, but not for other types of malignant pain. Analgesic efficacy data were extractable from only two of these studies, but were not combinable because one was a single-dose trial [27] and the other a multiple-dose trial [25]. The single-dose with ketoprofen study crossover reported a mean NSAID-induced PPID of 40% to 55% and SPID of 34% to 45%. The NSAID PPID in the multiple-dose study with naproxen 275 mg versus 550 mg was 23% to 33% [24].
McNicol and colleagues performed a Cochrane review and evaluated forty-two trials involving 3084 patients were included. Clinical heterogeneity of study methods and outcomes precluded meta-analyses and only supported a qualitative systematic review [28]. Sixteen studies lasted 1 week or longer and 11 evaluated a single dose [28]. They concluded that based upon limited data, NSAIDs appear to be more effective than placebo for cancer pain (7 out of 8 papers); clear evidence to support superior safety or efficacy of one NSAID over another is lacking; and trials of combinations of an NSAID with an opioid have disclosed either no difference (4 out of 14 papers), a statistically insignificant trend towards superiority (1 out of 14 papers), or at most a slight but statistically significant advantage (9 out of 14 papers), compared with either single entity. The short duration of studies undermines generalization of their findings on efficacy and safety of NSAIDs for cancer pain [28].
Cyclooxygenase (COX)-2 inhibitors may in theory be of greater therapeutic potential in well selected patients due to their anti-tumor/antiangiogenic properties [29]; especially in patients at high risk of gastrointestinal complications and those at risk of bleeding. Studies have shown in the sarcoma model of bone cancer pain that chronic inhibition of COX-2 activity with selective COX-2 inhibitors resulted in significant attenuation of bone cancer pain behaviors [both spontaneous and movement-evoked pain] as well as many of the neurochemical changes suggestive of both peripheral and central sensitization [30]. Microsomal prostaglandin E synthase-1 (mPGES-1) acts on COX-2 derived endoperoxide PGH2 to catalyze its isomerization PGE2. Thus, mPGES-1 inhibition may represent a therapeutic target to treating painful osseous metastases [31]. Lumiracoxib (Cyclooxygenase-189; Prexige®), is a highly selective COX-2 inhibitor which is not approved in the United States, Canada, Australia, England, and in some other countries due to hepatic related adverse events. Compared with diclofenac, lumiracoxib has substantially reduced affinity for COX-1, being 300-fold less potent. The pKa of lumiracoxib is 4.3 and thus, lumiracoxib is predicted to be more effective in a low pH environment; which may potentially be beneficial for pain relief in sites of metastatic bone lesions, where the local environment is acidic in nature.
Corticosteroids are commonly used for bone-related pain management which includes dexamethasone, methylprednisolone, and prednisone. Dexamethasone is often preferred orally because of its relatively high anti-inflammatory potency and low mineralocorticoid potency; therefore, dexamethasone has a lower risk of causing salt and water retention compared to equipotent doses of other corticosteroids. The mechanism of action of corticosteroid-induced analgesia is uncertain but may be related to decreasing tumor-related edema or inhibition of prostaglandin and leukotriene synthesis.
To date, only one study specifically addressed corticosteroid use for cancer-related bone pain. This study was a 14-day, randomized, double-blind, placebo-controlled, crossover study comparing 32 mg of oral methylprednisolone (MP) (16 mg twice daily) with placebo for symptoms in terminally ill cancer patients. After the initial 14 days, patients were continued on MP for a treatment period of 20 days. Pain intensity was significantly lower after MP compared with baseline or placebo in the crossover phase; 68% of patients responded that their pain control was better with MP compared to placebo by the end of the treatment phase. Furthermore, the findings of this study suggest that MP exerts its action rapidly, and the chances of obtaining better responses after 5 days of treatment are poor [32].
Animal models of cancer pain have demonstrated that peripheral nerve destruction can take place in both skin [33] and bone [34]. Additionally, sensitization of unmyelinated primary afferent fibers and damage to small and medium sized sensory neurons may occur. Metastatic tumor cells and/or tumor stromal cells in bone appear to lead to sensory nerve injury as evidenced by changes that include: sprouting of sensory fibers into bone [35], increased expression of activating transcription factor-3 (ATF-3) in the nucleus of sensory neurons that innervate bone, as well as up-regulation of galanin and glial fibrillary acidic protein with hypertrophy of satellite cells surrounding ipsilateral dorsal roof ganglion (DRG) sensory neuron cell bodies and ipsilateral DRG macrophage infiltration [36].
Gabapentin and pregabalin are voltage-gated calcium channel blockers believed to exert their effects at the alpha-2-delta-1 subunit. It has been demonstrated in animal studies, that gabapentin reverses dorsal horn changes associated with POM resulting in relief of spontaneous and movement-related pain [37]. In a sarcoma model chronic treatment with gabapentin did not affect tumor growth, tumor-induced bone destruction or tumor-induced neurochemical reorganization in sensory neurons or spinal cord, but did attenuate both ongoing and movement-evoked bone cancer-related pain behaviors [34]. These changes suggest that there is likely a neuropathic component which exists in conjunction with nociceptive and inflammatory components in painful osseous metastases. Stimulated by favorable effects of gabapentin in animal models demonstrated modulation of continuous and stimulus-related bone pain [37] and by the observation that gabapentin is reported to be useful for the treatment of neuropathic cancer pain [38], and as a synergistic adjuvant to opioid analgesics; Caraceni and colleagues published an anecdotal report describing their treatment of six consecutive patients with incident pain caused by bone metastases with gabapentin not completely controlled by opioid medication [39]. The addition of gabapentin was associated with significant clinical improvement of pain at rest and incident pain exacerbated by movement, which was sustained for up to 3 months [39]. As far as therapeutic agents in the class of "anticonvulsants", it is conceivable that topiramate may be an antiepileptic drug which is particularly well-suited for the treatment of painful osseous metastases, since in addition to its multiple mechanisms of action, it also possesses actions as a carbonic anhydrase inhibitor. Topiramate is a calcium channel blocker, sodium channel blocker, glutamic acid inhibitor; GABA facilitator and may affect the NMDA receptor complex. Adequate hydration is recommended due to the potential formulation of calcium phosphate renal stones.
One of the major classes of agents for the pharmacologic management of POM is that of opioid analgesics. Preclinical research suggests that there may be varying efficacy for different opioids [40], however, clinically there does not appear to be any opioid that is better than any other opioid for the treatment of painful osseous metastases. Although some opioids may provide more analgesia than other opioids for a specific individual patient, currently, "trial and error" is the only way to determine this. Opioids are considered an effective therapy for background pain in CIBP. However, their usefulness in breakthrough pain is less clear. It appears to be vitally important to match the characteristics of the opioid utilized to treat BTP; to the type of BTP experienced. Immediate release oral morphine has, at best, an onset of action of about 30 min [41]. This means that in patients with rapid-onset, short duration breakthrough pain, immediate release morphine will probably be ineffective. Furthermore, titration of opioids to doses that control episodes of breakthrough pain may result in unacceptable opioid side-effects [42]. Newer, rapid-onset opioids have been developed with the aim of mirroring the temporal features of breakthrough pain.
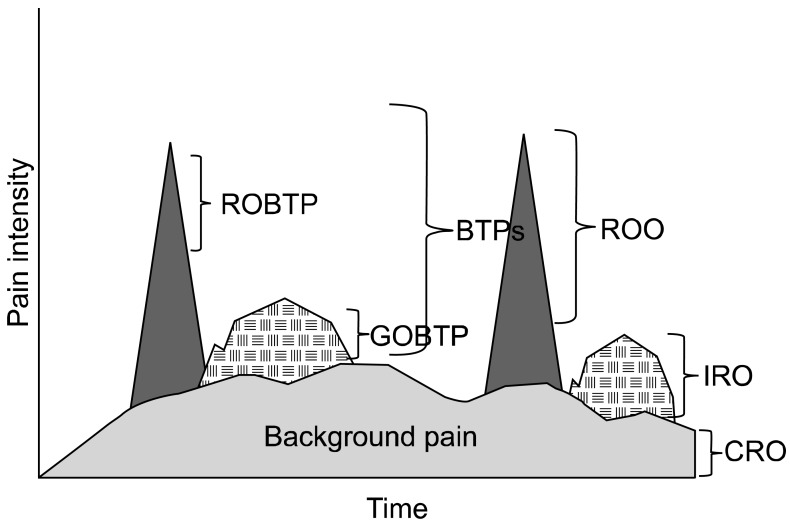
The author suggests a "triple opioid therapy approach" to using opioid analgesics to treat painful osseous metastases. A triple opioid therapy approach utilizes three different opioid formulations (a controlled release opioid, an immediate release opioid, and a rapid onset opioid). Enteral or transdermal extended release or controlled release opioids are employed for "maintenance" therapy to control the baseline or background constant pain. The patient receiving TOT then evaluates BTP episodes; 1) if a BTP episode seems relatively predictable and gradually intensifies over a half-hour or more Gradual Onset Breakthrough Pain then it may be treated early with an immediate release opioid formulation, however, 2) if a BTP episode is unpredictable and/or the intensity suddenly increases rapidly Rapid Onset BTP, then it should be treated with a rapid-onset opioid (Fig. 4).
Rapid-onset opioids FDA approved in the United States include: oral transmucosal fentanyl citrate [Atiq®], fentanyl buccal tablet [Fentora®], fentanyl buccal soluble film [Onsolis®], sublingual fentanyl [Abstral®], and fentanyl pectin nasal spray [Lazanda®]. Potential future rapid-onset opioids may include: intranasal fentanyl spray [Instanyl®] and fentanyl dry powder intrapulmonary inhaler [TAIFUN®].
Early-generation bisphosphonates (i.e., clodronate and etidronate) lack nitrogen and adhere to bone, where they are metabolized by osteoclasts. Metabolic products include cytotoxic ATP analogs that interfere with mitochondrial membrane potential and lead to osteoclast apoptosis [43]. Later generation, nitrogen-containing bisphosphonates (i.e., pamidronate, ibandronate and zoledronate) inhibit osteoclasts by a different mechanism. They are internalized - but not metabolized - by osteoclasts, where they subsequently inhibit an enzyme called farnesyl pyrophosphate (FPP) synthase. FPP synthase is required for producing intermediates (e.g. isoprenoid lipids) necessary for post-translational modification (prenylation) of several small GTPases, including Ras, Rho and Rac. These small GTPases are required for proper cellular vesicle transport, without which osteoclasts cannot form the tight sealing zones or ruffled borders at the bone surface that are required for resorption [43]. Additionally, nitrogen-contain bisphosphonates may lead to the accumulation of ispentyl pyrophosphate (IPP) which may be conjugated with adenosine monophosphate (AMP) to form an endogenous ATP analog triphosphoric acid 1-adenosin-5'-ylster 3-(3-methylbut 3-enyl) ester [ApppI] which may inhibit mitochondrial adenine nucleotide translocase (ANT) and cause osteoclast apoptosis [44]. In the United States bisphosphonates used for osteoporosis include zoledronic acid (indicated for a range of solid tumors, with osseous metastases---breast, prostate, non-small cell lung, renal, and others), pamidronate (included for breast cancer and multiple myeloma), ibandronate (indicated for breast cancer), and clodronate (not approved in U.S.).
Multiple studies have demonstrated the efficacy of bisphosphonates in reducing skeletal complications and pain from bone metastases [45,46]. Intravenous zoledronic acid has demonstrated the broadest clinical activity [47]. Zoledronate (zoledronic acid) is the most potent of the nitrogen containing bisphosphonates, displaying superior efficacy in inhibiting FPP synthase activity, reducing bone resorption and relieving pain when compared with other bisphosphonates, such as clodronate and pamidronate [48,49]. Zoledronic acid is the only bisphosphonate that has statistically shown significant reductions in skeletal morbidity, including bone pain, in patients with metastatic prostate cancer [50]. Fulfaro and colleagues demonstrated a relationship between a decrease in bone pain in 75% of patients and modification of C-telopeptide levels was identified in bone metastases from prostate cancer treated with zoledronic acid [51].
Zoledronate, in particular, has been reported to have direct antitumor properties in preclinical studies. It is capable of inducing tumor cell apoptosis [52], inhibiting cancer cell invasion [53] and limiting metastatic outgrowth in visceral tissues at extremely high doses [49]. Zoledronate treatment has been associated with a decline in circulating levels of the potent pro-angiogenic molecule, VEGF, in cancer patients [54]. Zoledronate-mediated reductions in VEGF levels were associated with increased time to a skeletal-related event, increased time to the progression of bone disease and longer time to the worsening of performance status [55]. Zoledronic acid distributes and bonds to osseous tissues and has a triphasic post-infusion decline process with a terminal half-life of 146 hours. Prior to therapy initiation of zoledronate, a dental evaluation and subsequent follow-up are needed in efforts to monitor for the occurrence and risk of osteonecrosis of the jaw.
Zoledronic acid can cause flu-like symptoms that are manageable with standard treatment. Renal monitoring is recommended due to association with iatrogenic renal function deterioration. Use of zoledronic acid should be avoided in patients with a Clcr of ≤ 30 ml/min and caution should be utilized when using coledronate in patients with other nephrotoxic agents. Dose reductions should be followed according to the package information sheet for patients with renal dysfunction.
Only certain types of cancers (e.g. breast cancer, prostate cancer) may respond in some fashion to hormonal therapy. Intuitively, it would seem that any hormonal therapy which achieves antineoplastic results may also possess antinociceptive qualities under certain circumstances. An example of a cancer type which may respond to hormonal therapy is prostate cancer. Androgen deprivation therapy is achievable with surgical castration (bilateral orchiectomy), or medical castration which may include agents such as: synthetic gonadotropin releasing hormone (GrRH) agonists (e.g. leuprolide, buserelin, goserelin, histrelin, [triptorelin-in phase II trials is a 60 month formulation triptorelin embonate that is under development]), cytochrome P450 enzyme 17A1 (CYP17A1) inhibitors [inhibition of androgen synthesis] (e.g. nonselective CYP17A1 inhibitors) ketoconazole, [aromatase inhibitors] aminoglutethimide], selective CYP17A1 inhibitors [abiraterone acetate - in phase III clinical trials, TOK-001 and TAK-700 in phase I/II trials], androgen receptor antagonists (e.g. bicalutamide, nilutamide, flutamide, and [MDV 31000 - in phase III clinical trials, BMS-641988 in phase I clinical trials]), inhibitors of 5α-reduction [which converts testosterone to the more potent dihydrotestosterone] (e.g. finasteride, dustasterude).
Androgen-deprivation therapy has become a vital component of treatment for certain types of cancer (e.g. advanced prostate cancer). Gonadotropin-releasing hormone (GnRH) agonists override the normal pulsatile control of the pituitary by providing continuous stimulation with resultant down regulation of pituitary GnRH receptors with consequent reduction of luteninizing hotrmone (LH) and follicle-stimulation hormone (FSH) production and testosterone suppression. However, before this occurs, there tends to be a transient increase in LH, FSH and testosterone. GnRH antagonists (e.g. degarelix and abarelic) bind directly to pituitary GnRH receptors blocking the effects of GnRH on the pituitary with immediate suppression of LH, FSH, and testosterone.
External beam RT for osseous metastases may lead to improved analgesia, elimination or reduction of analgesic usage, functional improvement, such as increased ambulation, and reduction in the risk of fracture in weight-bearing bones. Large multiinstitutional randomized trials conducted by the Radiation Therapy Oncology Group have demonstrated that 80% of patients receiving RT for osseous metastases will experience complete to partial pain relief, typically within 10-14 days of the initiation therapy [56]. A correlation was also found between the incidence of pain relief and the site of bone metastases, in that a lower response was shown in limb localizations [57]. Dennis and colleagues found that patients suffering from painful bone metastases with an estimated survival of 3 months should still be considered for palliative radiotherapy [58].
Approximately 80% of patients may be successfully treated with sequential whole-skeleton radiation, in which 6-8 Gy is administered as a single fraction to either the upper and lower part of the body, followed by a second dose of 6-8 Gy, given 4-6 weeks later, to the remainder of the body [59]. Most prospective randomized trials evaluating differences in the outcomes have shown that single fraction regimens (mostly 8 Gy) are at least equal in analgesic efficacy to the various fractionated regimens [60]. These results have been confirmed in three meta-analyses [61-63]. Wu et al. [61] included eight randomized trials (3,260 patients) in a meta-analysis, comparing 1 × 8 Gy single fraction radiotherapy with various multi-fraction regimens and found that all multi-fraction regimens were essentially equal to single fraction therapy.
Similar results have been observed in the meta-analysis of Sze et al. [62], which included 3,621 patients from 12 randomized trials. The complete response rates were 34% (508/1,476) after single-fraction radiotherapy and 32% (475/1,473) after multi-fraction radiotherapy (0dds ratio [OR] 1.10; 95% CI 0.94-1.30, P > 0.05). Overall response rates were 60% (1,080/1,814) and 59% (1,060/1,807), respectively (OR 1.03; 95% CI 0.90-1.19; P < 0.05) [62,64]. Chow and colleagues included 5,000 patients from 16 randomized trials in their meta-analysis [63]. The overall response rates (intention-to-treat analysis) were 58% (1,468/2,513) after single-fraction radiotherapy (mostly 1 × 8 Gy) and 59% (1,466/2,487) after multi-fraction radiotherapy (mostly 5 × 4 Gy or 10 × 3 Gy) (OR 0.99; 95% CI 0.95-1,03; P = 0.60) [63,64].
Nomiya and colleagues analyzed the time course of pain relief by radiotherapy for cancer pain [65]. Complete pain relief was obtained in 45/91 (49%) cases, and partial (> or = 50%) pain relief was obtained in 83/91 (91%) cases. The mean time to obtain 50% pain relief was 13 days. The mean time to obtain complete pain relief (n = 45) was 24 days [65].
Huisman and colleagues performed a systematic review in which 10 of 707 articles were selected for inclusion and seven entered a meta-analysis [66]. Overall, the study quality was mediocre. Of the 2,694 patients initially treated for metastatic bone pain, 527 (20%) patients underwent reirradiation. Overall, a pain response after reirradiation was achieved in 58% of patients (pooled overall response rate 0.58, 95% confidence interval = 0.49-0.67) [66]. Reirradiation of painful bone metastases was found to be effective in terms of pain relief for a small majority of patients; approximately 40% of patients do not benefit from reirradiation [66].
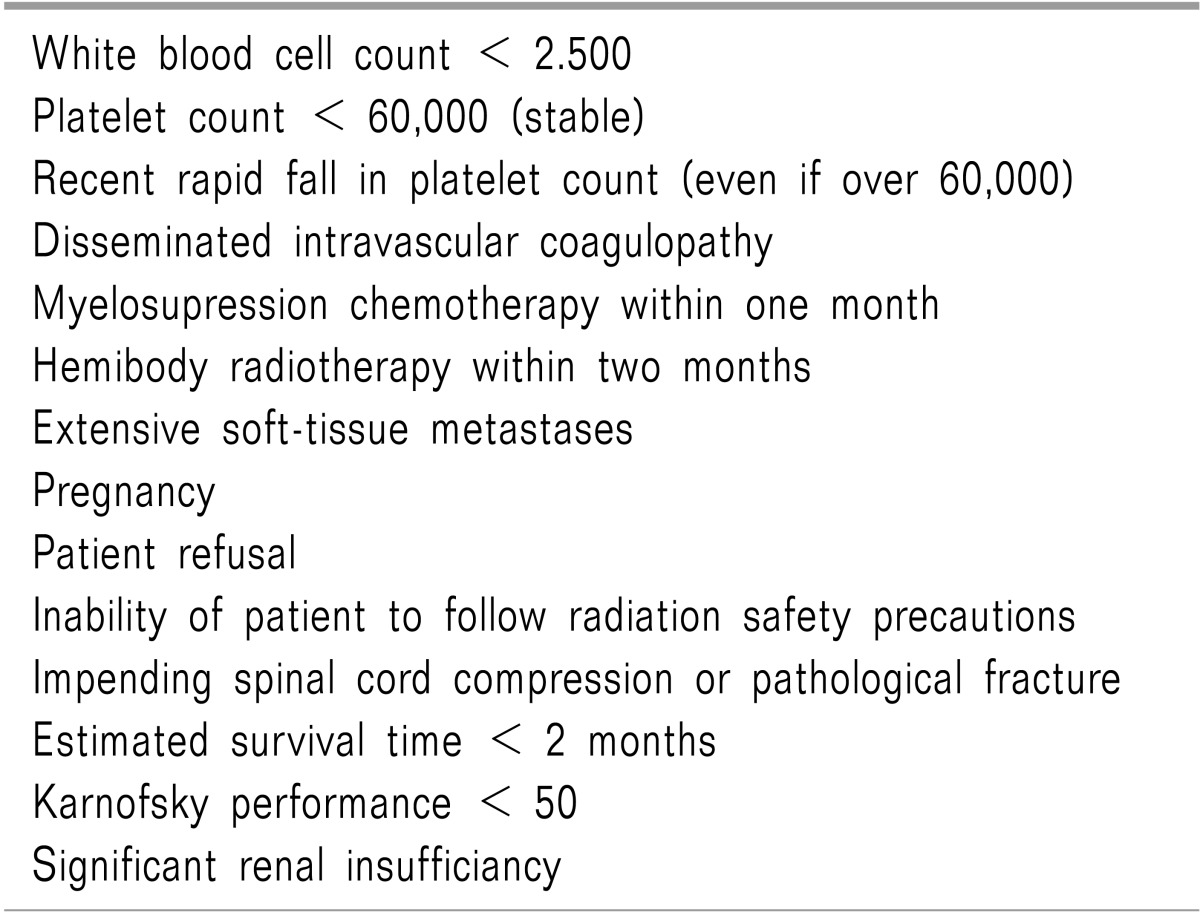
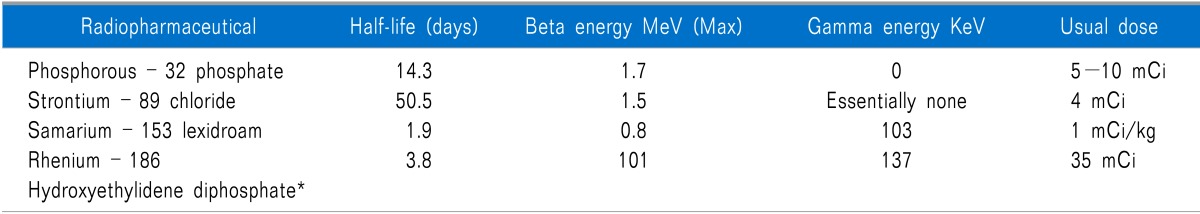
Radiopharmaceuticals provide several advantages over conventional external beam radiotherapy: 1) they can be administered intravenously, 2) they can treat multiple, diffuse sites with mild bone marrow depression, and 3) they cause fewer adverse side-effects such as nausea, vomiting, diarrhea, and tissue damage [67]. Radiopharmaceuticals are relatively easy to administer but should be performed by clinicians appropriately trained in nuclear medicine. Although the preparation and steps for each patient surrounding radiopharmaceutical administration is different and should be individualized; certain common treatment guidelines exist (Table 2). Absolute contraindications for using radiopharmaceuticals include pregnancy and patient refusal. Relative contraindications require careful consideration of risks versus potential benefits within the context of the patients' wishes (Table 3) [67]. Multiple radiopharmaceuticals exist which may provide analgesia from painful osseous metastases, some agents have appropriate energies to be imaged as well (Table 4).
Figuls and colleagues updated a Cochrane Review to determine efficacy and safety of radioisotopes in patients with painful bone metastases. Their update includes 15 studies (1,146 analyzed participants): four (325 participants) already included and 11 new (821 participants). They found a small benefit of radioisotopes for complete relief (risk ratio (RR) 2.10, 95% CI 1.32 to 3.35; Number needed to treat to benefit (NNT) = 5) and complete/partial relief (RR 1.72, 95% CI 1.13 to 2.63; NNT = 4) in the short and medium term (eight studies, 499 participants). Leucocytopenia and thrombocytopenia are secondary effects significantly associated with the administration of radioisotopes (RR 5.03; 95% CI 1.35 to 18.70; Number needed to treat to harm (NNH) = 13). Pain flares were not higher in the radioisotopes group (RR 0.74; 95% CI 0.27 to 2.06) [68].
Strontium is a divalent cation, like calcium, and is incorporated into hydroxyapatite in the bone after intravenous injection and is a bone specific radiosotope. Sr-89-chloride (Metastron; GE Healthcare Global, Bucks, UK) was the first FDA-approved radiopharmaceutical for bone pain palliation [69].
Pain relief usually begins within two weeks of treatment, with maximum benefit by six weeks, and lasts between four and 15 months [70]. Mild thrombocytopenia or leukopenia may occur in up to 80 percent of patients [70]. Platelets decline about 15-30 percent below pretreatment levels and usually completely recover in two to three months, enabling repeat treatment at that time [70]. Occasionally, recovery of platelet count to baseline may take about six month [70]. In addition, 15-20 percent reductions in WBCs have also been recorded following 89Sr administration [70]. A transient flushing sensation immediately after rapid 89Sr injection has been noted and is self limited. Bone pain may transiently increase in some patients (≤ 20% reported).
A recent systematic review of the available literature published by Finlay et al. showed a percentage of complete responders to Sr-89 ranging from 8% to 77%, with a mean value of 32%, and no responders ranging from 14% to 52% (mean, 25%). In general, 44% of patients had some degree of response to Sr-89 treatment, giving a mean overall response of 76%) [71].
Samarium-153 lexidronam (153Sm-EDTMP) was originally described by William Goeckler PhD in 1984, and it was approved by the Federal Drug Administration (FDA) on March 28, 1987 for relief of pain in patients with osteoblastic bone metastases [72]. 153Sm-EDTMP is a stable complex of radioactive samarium-153 and ethylene diamine tetramethylene phosphonic acid [73]. Sartor and colleagues reported the safety and efficacy of repeated doses of Sm-153 in patients with metastatic bone pain [74]. Significant decreases in pain scores (P < 0.002) were observed at week 4 after each of the first 3 doses and maintained at week 8 after the first 2 doses (P < 0.003) but not after the third dose. Decreases in pain scores were observed in 70%, 63%, and 80% of patients, respectively, at week 4 after the first 3 administrations.
Patients may be offered focal ablative therapy (radiofrequency ablation [RFA] [75] or cryoablation) for painful metastases when 3 factors are present. First, a patient reports moderate or severe pain, typically ≥ 4 of 10 for worst pain in a 24-hour period. Second, a patient's local pain is limited to 1 or 2 sites and the patient's pain is associated with a corresponding abnormality evident with cross-sectional imaging. Third, treatment of the patient's painful metastatic lesion must be amenable to the use of ablative devices. Lesions that amenable to ablative therapy are typically osteolytic or mixed osteolytic/osteoblastic in nature or otherwise composed of soft tissue [76]. Exclusion of patients from focal ablative therapy usually occurs when one or more of the following situations are present. First, if a successful treatment requires the treatment of a portion of the lesion located within 1 cm of the spinal cord, major motor nerve, brain, artery of Adamkiewicz, bowel, or bladder [76]. Di Staso et al. suggest that radiofrequency ablation (RFA) followed by radiotherapy (RT) (RFA-RT) is safe and more effective than RT alone [77].
While cryoblation may effectively treat intact or sclerotic bone, RFA energy is poorly delivered into sclerotic or otherwise intact bone [78]. Cryoablation may have several other unique advantages over RFA for treatment of pain due to metastatic disease. Importantly, the zone of ablation is readily monitored with intermittent CT or MR imaging. The ice ball that is generated appears as a low attenuation region with a well-defined margin with CT and with various pulse sequences with MR imaging [76]. Cryoablation also allows the simultaneous use of multiple cryoprobes, which allows complete ablation of large lesions (up to approximately 8-cm diameter) in a single session. This approach avoids leaving residual neoplasm between the separate cryoprobes that is possible between sequential single overlapping ablations [76]. Furthermore, cryoblation may treat larger lesions than RFA, since the site of the ice ball generated is generally larger than the tip of the radiofrequency probe.
The incidence of spinal metastases and vertebral compression fractures continues to rise, with associated axial pain, progressive radiculopathy/myelopathy, and mechanical instability. Vertebral augmentation procedures such as percutaneous vertebroplasty and percutaneous kyphoplasty can provide relief in patients with pathologic vertebral body compression fractures that do not cause neurological deficits but severely compromise quality of life largely because of intractable pain, but also due to loss of independence, mobility, and function often with resulting isolation/loneliness [79].
Percutaneous vertebroplasty, first described in 1987, is a radiologically guided procedure in which percutaneous injection of polymethylmethacrylate, a surgical bone cement, is injected into a vertebra under imaging guidance [80]. Lee and colleagues reported on 19 percutaneous vertebroplasty procedures performed mainly in breast, prostate, lung and renal cancers [81]. Of these 19 cases, 10 patients (53%) were treated for solitary lesions, 3 (16%) were injected at two levels and the remaining 6 cases (31%) underwent cement injection at three levels. The majority of individuals (84%) reported short- and long-term symptomatic improvements [81]. Saliou et al. evaluated a total of 74 vertebrae in 51 patients, (22 women and 29 men) with a mean age of 62.5 years with malignant fractures of the spine with epidural involvements [82]. They concluded that percutaneous vertebroplasty provided effective analgesia in patients experiencing pain related to malignant spinal tumors with epidural extension, and was associated with a relatively low complication rate [82].
Kyphoplasty has evolved from vertebroplasty and aims to offer the benefit of analgesia in vertebral fractures in combination with restoration of vertebral body height. A balloon-like device is inflated, which restores vertebral body height and creates a cavity into which cement is then injected [83].
Qian and colleagues performed a retrospective review of clinical outcome data for 48 patients with multiple spinal metastases treated with kyphoplasty [84]. Outcome data (vertebral body height variation, degree of kyphosis, visual analog scale score for pain, Oswestry Disability Index score, the Short Form-36 [SF-36] questionnaire score for function) were collected preoperatively, postoperatively, and at 1 month, 6 months, 1 year, and 2 years after treatment. The mean visual analog scale score decreased significantly from presurgery to postsurgery (7.4 ± 2.1 to 3.8 ± 1.6; P < 0.001), as did the Oswestry Disability Index score (71.5 ± 16.7 to 32.4 ± 9.6; P < 0.001). The SF-36 scores for bodily pain, physical function, vitality, and social functioning all also showed significant improvement (P < 0.05). Qian et al. concluded that kyphoplasty appears to be an effective, minimally invasive procedure for the stabilization of pathological vertebral fractures caused by metastatic disease, even in levels with vertebral wall deficiency [84].
The use of intrathecal analgesics is an important treatment consideration for many patients with chronic cancer pain. Intrathecal analgesia has emerged as a key therapeutic option for pain relief for patients who have failed other treatment avenues as well as patients with adequate analgesia on high dose enteral or parenteral therapy but with unacceptable side effects.
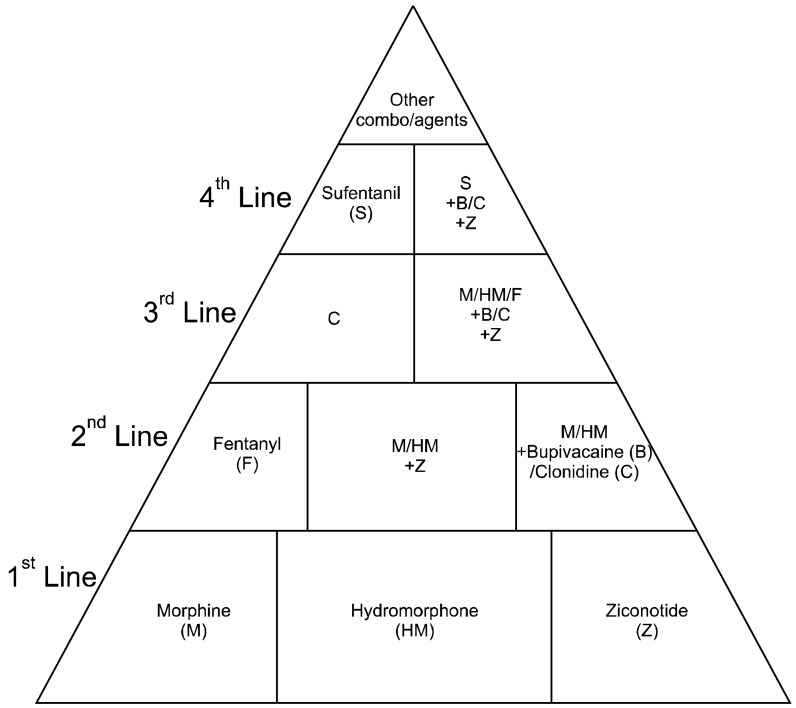
Smith and colleagues performed a multicenter randomized, prospective trial evaluating intrathecal drug delivery for 202 cancer patients [85]. Specific outcomes from the Smith et al. study were that opioid-induced toxicities such as fatigue, sedation, and cognitive slowing were improved compared with patients receiving comprehensive medication management. Pain scores were also improved with respect to baseline and compared with the scores in patients receiving comprehensive medication management, with nearly 2/3 Implantable Drug Delivery System (IDDS) patients, having scores in the target range of less than 4/10. The number of intrathecal drug choices are limited and should be guided by consensus guidelines [86]. First-line intrathecal analgesics include morphine, sulfate, hydromorphone and ziconotide [86], however, there are other alternative agents as well (Fig. 5) [86]. Appropriate selection of patients with intractable cancer pain for chronic intrathecal analgesia therapy is paramount [87] and clear communication of the rationale for infusion is very important, as is regular education about infusion management.
The RANK-RANKL system plays a fundamental role in the maturation and function of osteoclasts and thus in the development and progression of bone metastasis. Therefore, inhibition of this system has been evaluated as therapeutic target for the treatment of osteolytic diseases, including bone metastasis [88].
It appears that some of the pain from metastatic bone lesions may be secondary to the effects of osteoclastic activity, so that "shutting down" osteoclastic activity is paramount to incorporate in analgesic treatments. Osteoclast bone-resorbing activity is dependent on the binding of the TNF family molecule osteoprotegerin ligand (OPGL) [89], which is expressed on activated T cells and osteoblasts, to a receptor termed receptor activator of nuclear factor kB (NF-kB), abbreviated RANK [89]. RANK is expressed on osteoclast precursors and mature osteoclasts [90]. Any treatment that impedes the OPGL-RANK interaction will impair RANK activation and therefore impair osteoclastic activity and bone resorption. Osteoprotegerin (OPG) is a soluble tumor necrosis factor receptor molecule that is secreted and binds to the RANK activating site of OPGL, acting as a "dummy" or "decoy" receptor and preventing OPGL from binding to and activating the osteoclast RANK receptor [89] (Fig. 2).
Amgen created a recombinant Fc-OPG (AMGN-0007) to treat multiple myeloma and bone metastatic breast cancer. Results from the Phase I trial were encouraging, in that Fc-OPG was well tolerated and its inhibitory effects on bone resorption were similar to the bisphosphonate, pamidronate [91]. However, due to the superior efficacy of their newer agent, denosumab (AMG-162) - a fully human monoclonal antibody that specifically neutralizes RANKL - thereby inhibiting bone resorption, and concerns regarding deleterious OPG-mediated protection from TRAIL mediated apoptosis in cancer cells, Amgen ceased further clinical development of AMGN-0007 [92].
Purinergic modulators appear to have the capacity to affect nociceptive processes.
Kaan et al. utilized a rat model of bone cancer with MRMT-1 carcinoma cells and demonstrated that pain-related behaviors were increased and phosphorylation of ERK 1/2 (p-ERK 1/2) protein expression levels were increased in the spinal dorsal horn and DRG of the CIBP group relative to the sham group [93]. Using AF-353, an orally administered potent and selective P2X3 and P2X2/3 receptor antagonist, Kaan and colleagues demonstrated attenuation of bone cancer pain-related behaviors, reduced bone cancer-induced dorsal horn neuronal hyperexcitability in vivo, and reduced carcinoma cells-induced extracellular signal-regulated kinase activation (p-ERK 1/2) in dorsal root ganglion neurons [93].
Additionally, Chen et al. published that P2Y1R mRNA and phosphorylated ERK1/2 (p-ERK1/2) protein expression levels were increased in the spinal dorsal horn and DRG of the CIBP group relative to the sham group [94]. Intrathecal injection of the P2Y1R antagonist MRS2179 decreased P2Y1R mRNA and p-ERK1/2 protein expression in the spinal dorsal horn and DRG (P < 0.01), as well as pain-related behavior including tactile allodynia, spontaneous pain, and ambulatory-evoked pain [94]. These results provide supporting evidence that the inhibition of P2Y1R-mediated ERK1/2 phosphorylation in the spinal dorsal horn and DRG can attenuate nociception transmission [94].
Additionally, it is conceivable that potential future therapeutic agents may include cannabinoid receptor modulators, nerve growth factor modulators, inhibitors of cathepsin K, Src inhibitors, inhibitors of MMP-9 αVβ3 antagonists, CXCR4 antagonists, endothelin-A receptor antagonists (e.g. atrasentan), and tyrosine kinase inhibitors (e.g. cabozantinib).
T-cell death-associated gene 8 (TDAG8) is a G-protein-coupled receptor (GPCR) belonging to the ovarian cancer G-protein-coupled receptor 1 subfamily of proton-sensing and psychosine-sensitive receptors [95]. Recently, expression of TDAG8 has been shown in spinal cord and dorsal root ganglion (DRG), supporting the possible involvement of TDAG8 in nociception [96]. Chen et al. (2009) have shown that TDAG8 participates in complete Freund's adjuvant-induced chronic inflammatory pain [97]. Tissue acidosis is an important feature of cancer [98]. It has been suggested that a relatively high proton concentration, known as acidosis, is direct link between disease and pain [99]. TDAG8 is a proton sensing receptor but whether it is involved in bone cancer pain remains unclear.
In vitro research has demonstrated that TDAG8 can be coupled to Gs protein, which stimulates adenylyl cyclase, leading to cAMP response element-dependent transcription via protein kinase A (PKA) phosphorylation of the cAMP response element-binding (CREB) protein [100].
Hang et al. demonstrated the relationship between TDAG8 expression and the initiation and maintenance of CBP. Activation of spinal TDAG8 contributes to CBP through the PKA signaling pathway in rats. A bone cancer pain model was made by inoculation of Walker 256 cells into the intramedullary space of rat tibia. Intrathecal TDAG8 siRNA attenuated bone cancer pain behaviors during the initiation and maintenance phases; there were also concomitant decreases in TDAG8 mRNA and protein levels in spinal cord. On days 6, 12 and 18 after inoculation, the relative levels of spinal TDAG8 mRNA significantly and time dependently increased in BCP rats compared to sham and NS rats [101]. The upregulation of TDAG8 protein was also demonstrated by Western blot analysis and immunohistochemistry. Western blot analysis showed that on days 6, 12 and 18 after inoculation, a statistically significant increase in TDAG8 protein levels was observed in BCP rats compared to sham and NS rats. Moreover, they found spinal PKA and phosphorylated cAMP response element-binding (pCREB) protein levels were up-regulated in the rat model of bone cancer pain. Knockdown of TDAG8 resulted in reduced bone cancer pain-induced spinal PKA and pCREB protein expression in two procedures. Furthermore, intrathecal H-89 (a PKA inhibitor) significantly attenuated bone cancer pain behaviors in rats [101].
Lozano-Ondoua and colleagues demonstrated that CB(2) agonists reduce breast cancer-induced bone pain, bone loss and breast cancer proliferation via cytokine/chemokine suppression [102]. Studies utilized the spontaneously-occurring murine mammary cell line (66.1) implanted into the femur intramedullary space; measurements of spontaneous pain, bone loss, and cancer proliferation were made. The systemic administration of a CB(2) agonist, JWH015, for 7 days significantly attenuated bone remodeling, assuaged spontaneous pain, and decreased primary tumor burden. CB(2)-mediated effects in vivo were reversed by concurrent treatment with a CB(2) antagonist/inverse agonist but not with a CB(1) antagonist/inverse agonist. In vitro, JWH015 reduced cancer cell proliferation and inflammatory mediators that have been shown to promote pain, bone loss, and proliferation. Taken together, these results suggest CB(2) agonists as a novel treatment for breast cancer-induced bone pain, in which disease modifications include a reduction in bone loss, suppression of cancer growth, attenuation of severe bone pain, and increased survival without the major side effects of current therapeutic options [102].
Utilizing a cancer-induced bone pain (CIBP) model, induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells; Hu and colleagues demonstrated that: 1) Intrathecal injection with the same dose (0.3 nmol) of lipoxin A4 (LXA4), lipoxin B4 (LXB4) or aspirin-triggered-15-epi-lipoxin A4 (ATL) could alleviate the mechanical allodynia in CIBP on day 7 after surgery. ATL showed a longer effect than the others and the effect lasted for 6 hours. ATL administered through i.v. injection could also attenuate the allodynia in cancer rats. Additionally, real-time PCR analysis revealed that, compared with vehicle, i.t. injection with ATL could significantly attenuate the expression of the mRNA of proinflammatory cytokines (IL-1β and TNF-α) in the spinal cord in CIBP. These data suggested that LXs and analogues exert strong analgesic effects on CIBP. These analgesic effects in CIBP are associated with suppressing the expression of spinal proinflammatory cytokines [103].
Metastatic disease to the bone has been a crippling devastating complication of various cancers, leaving patients bedridden or wheelchair-bound and victims of suffering with unbearable pain. Knowledge surrounding the pathophysiology of painful osseous metastases is rapidly changing. Treatment approaches continue to be introduced into practice as they are approved. The advent of intravenous bisphosphonates has not only given clinicians another agent to reduce pain but also to reduce and/or postpone the risk of "skeletal-related events". RANK-L inhibition with denosumab represents a new therapeutic approach to also prevent or delay "skeletal-related events" as well as reduce pain. A greater understanding of the pathophysiology of painful osseous metastases may lead to improved analgesia with minimal adverse effects by utilizing tailor-made selective targeted therapy. It is hoped that potential future therapeutic agents for the treatment of painful osseous metastases may revolutionize current pharmacologic approaches and lead to improved patient outcomes with better quality of life.
I am deeply indebted to deceased Dr. Smith who have been worked as Editor-in-Chief of Pain Physician as well as an editorial board member of The Korean Journal of Pain. He had an incredible wealth of knowledge and left tremendous achievements including books and papers in the field of pain medicine. This article shares many similarities, including tables and figures, with a review article published by Dr. Smith. (Smith HS. Painful osseous metastasis. Pain Physician 2011: 14; E373-E405). This review article is published in honor and remembrance of Dr. Smith with permission from Pain Physician. - Francis Leem, Editor-in-Chief of The Korean Journal of Pain.
ACKNOWLEDGEMENTS
The authors would like to thank Pya Seidner for her enormous assistance in the preparation of this manuscript.
References
1. Boyle P, Levin B. International Agency for Research on Cancer. World Health Organization. World cancer report 2008. Lyon: International Agency for Research on Cancer;2008.
2. Berruti A, Dogliotti L, Bitossi R, Fasolis G, Gorzegno G, Bellina M, et al. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: predictive role of bone resorption and formation markers evaluated at baseline. J Urol. 2000; 164:1248–1253. PMID: 10992374.

3. Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001; 93:247–257. PMID: 11514084.

4. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007; 18:1437–1449. PMID: 17355955.

5. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007; 132:312–320. PMID: 17916403.

6. Laird BJ, Walley J, Murray GD, Clausen E, Colvin LA, Fallon MT. Characterization of cancer-induced bone pain: an exploratory study. Support Care Cancer. 2011; 19:1393–1401. PMID: 20680354.

7. Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997; 69:1–18. PMID: 9060007.

8. Delaney A, Fleetwood-Walker SM, Colvin LA, Fallon M. Translational medicine: cancer pain mechanisms and management. Br J Anaesth. 2008; 101:87–94. PMID: 18492671.

9. Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 1998; 95:13453–13458. PMID: 9811821.

10. Everts V, Delaissé JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, et al. The bone lining cell: its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res. 2002; 17:77–90. PMID: 11771672.

11. Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983; 80:2752–2756. PMID: 6405388.

12. Josephsen K, Praetorius J, Frische S, Gawenis LR, Kwon TH, Agre P, et al. Targeted disruption of the Cl-/HCO3- exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci U S A. 2009; 106:1638–1641. PMID: 19164575.

13. Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000; 25:343–346. PMID: 10888887.

14. Karsdal MA, Henriksen K, Sørensen MG, Gram J, Schaller S, Dziegiel MH, et al. Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am J Pathol. 2005; 166:467–476. PMID: 15681830.

15. Baron R, Neff L, Louvard D, Courtoy PJ. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985; 101:2210–2222. PMID: 3905822.

16. Miyazaki T, Tanaka S, Sanjay A, Baron R. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006; 16:68–74. PMID: 16633924.

17. Bradt J, Dileo C, Grocke D, Magill L. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev. 2011; (8):CD006911. PMID: 21833957.

18. Jane SW, Chen SL, Wilkie DJ, Lin YC, Foreman SW, Beaton RD, et al. Effects of massage on pain, mood status, relaxation, and sleep in Taiwanese patients with metastatic bone pain: a randomized clinical trial. Pain. 2011; 152:2432–2442. PMID: 21802850.

19. Burton AW, Cleeland CS. Cancer pain: progress since the WHO guidelines. Pain Pract. 2001; 1:236–242. PMID: 17134407.

20. Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995; 63:65–76. PMID: 8577492.

21. Mercadante S. Intravenous morphine for management of cancer pain. Lancet Oncol. 2010; 11:484–489. PMID: 20434717.

22. Vielhaber A, Portenoy RK. Advances in cancer pain management. Hematol Oncol Clin North Am. 2002; 16:527–541. PMID: 12170566.

23. Urch C. The pathophysiology of cancer-induced bone pain: current understanding. Palliat Med. 2004; 18:267–274. PMID: 15198116.

24. Eisenberg E, Berkey CS, Carr DB, Mosteller F, Chalmers TC. Efficacy and safety of nonsteroidal antiinflammatory drugs for cancer pain: a meta-analysis. J Clin Oncol. 1994; 12:2756–2765. PMID: 7989953.

25. Levick S, Jacobs C, Loukas DF, Gordon DH, Meyskens FL, Uhm K. Naproxen sodium in treatment of bone pain due to metastatic cancer. Pain. 1988; 35:253–258. PMID: 3226754.

26. Stambaugh JE Jr, Drew J. The combination of ibuprofen and oxycodone/acetaminophen in the management of chronic cancer pain. Clin Pharmacol Ther. 1988; 44:665–669. PMID: 2461823.

27. Sacchetti G, Camera P, Rossi AP, Martoni A, Bruni G, Pannuti F. Injectable ketoprofen vs. acetylsalicylic acid for the relief of severe cancer pain: a double-blind, crossover trial. Drug Intell Clin Pharm. 1984; 18:403–406. PMID: 6373214.

28. McNicol E, Strassels SA, Goudas L, Lau J, Carr DB. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database Syst Rev. 2005; (1):CD005180. PMID: 15654708.

29. Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997; 99:2254–2259. PMID: 9151799.

30. Sabino MA, Ghilardi JR, Jongen JL, Keyser CP, Luger NM, Mach DB, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002; 62:7343–7349. PMID: 12499278.
31. Isono M, Suzuki T, Hosono K, Hayashi I, Sakagami H, Uematsu S, et al. Microsomal prostaglandin E synthase-1 enhances bone cancer growth and bone cancer-related pain behaviors in mice. Life Sci. 2011; 88:693–700. PMID: 21324324.

32. Bruera E, Roca E, Cedaro L, Carraro S, Chacon R. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep. 1985; 69:751–754. PMID: 2410117.
33. Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, et al. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci. 2001; 21:9367–9376. PMID: 11717370.

34. Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 2005; 193:85–100. PMID: 15817267.

35. Halvorson KG, Sevcik MA, Ghilardi JR, Rosol TJ, Mantyh PW. Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin J Pain. 2006; 22:587–600. PMID: 16926574.

36. Jimenez-Andrade JM, Mantyh P. Cancer pain: from the development of mouse models to human clinical trials. In : Kruger L, Light AR, editors. Translational pain research: from mouse to man. Boca Raton (FL): CRC Press;2010. p. 1–22.
37. Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005; 102:132–140. PMID: 15618797.

38. Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya Tur R, Maltoni M, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol. 2004; 22:2909–2917. PMID: 15254060.

39. Caraceni A, Zecca E, Martini C, Pigni A, Bracchi P. Gabapentin for breakthrough pain due to bone metastases. Palliat Med. 2008; 22:392–393. PMID: 18541644.

40. Kato A, Minami K, Ito H, Tomii T, Matsumoto M, Orita S, et al. Oxycodone-induced analgesic effects in a bone cancer pain model in mice. Oncology. 2008; 74(Suppl 1):55–60. PMID: 18758199.

41. Bailey F, Farley A. Oral opioid drugs. In : Davies A, editor. Cancer-related breakthrough pain. Oxford: Oxford University Press;2006. p. 43–55.
42. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999; 81:129–134. PMID: 10353500.

43. Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol. 2009; 6:163–174. PMID: 19190592.

44. Mönkkönen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsäläinen J, et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006; 147:437–445. PMID: 16402039.

45. Berenson JR, Hillner BE, Kyle RA, Anderson K, Lipton A, Yee GC, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002; 20:3719–3736. PMID: 12202673.

46. Pistevou-Gombaki K, Eleftheriadis N, Sofroniadis I, Makris P, Kouloulias V. Palliative treatment of painful bone metastases from non-Hodgkin lymphoma with disodium pamidronate. J Exp Clin Cancer Res. 2002; 21:429–432. PMID: 12385590.
47. Santini D, Fratto ME, Vincenzi B, Galluzzo S, Tonini G. Zoledronic acid in the management of metastatic bone disease. Expert Opin Biol Ther. 2006; 6:1333–1348. PMID: 17223741.

48. Amir E, Whyne C, Freedman OC, Fralick M, Kumar R, Hardisty M, et al. Radiological changes following second-line zoledronic acid treatment in breast cancer patients with bone metastases. Clin Exp Metastasis. 2009; 26:479–484. PMID: 19266291.

49. Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004; 10:4559–4567. PMID: 15240548.

50. Furlow B. Zoledronic acid palliation in bone-metastatic breast cancer. Lancet Oncol. 2006; 7:894. PMID: 17099983.

51. Fulfaro F, Leto G, Badalamenti G, Arcara C, Cicero G, Valerio MR, et al. The use of zoledronic acid in patients with bone metastases from prostate carcinoma: effect on analgesic response and bone metabolism biomarkers. J Chemother. 2005; 17:555–559. PMID: 16323446.

52. Rachner TD, Singh SK, Schoppet M, Benad P, Bornhäuser M, Ellenrieder V, et al. Zoledronic acid induces apoptosis and changes the TRAIL/OPG ratio in breast cancer cells. Cancer Lett. 2010; 287:109–116. PMID: 19577359.

53. Woodward JK, Neville-Webbe HL, Coleman RE, Holen I. Combined effects of zoledronic acid and doxorubicin on breast cancer cell invasion in vitro. Anticancer Drugs. 2005; 16:845–854. PMID: 16096432.

54. Santini D, Vincenzi B, Galluzzo S, Battistoni F, Rocci L, Venditti O, et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007; 13:4482–4486. PMID: 17671133.

55. Vincenzi B, Santini D, Dicuonzo G, Battistoni F, Gavasci M, La Cesa A, et al. Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res. 2005; 25:144–151. PMID: 15767788.

56. Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the study by the Radiation Therapy Oncology Group. Cancer. 1982; 50:893–899. PMID: 6178497.

57. Arcangeli G, Micheli A, Arcangeli G, Giannarelli D, La Pasta O, Tollis A, et al. The responsiveness of bone metastases to radiotherapy: the effect of site, histology and radiation dose on pain relief. Radiother Oncol. 1989; 14:95–101. PMID: 2469105.

58. Dennis K, Wong K, Zhang L, Culleton S, Nguyen J, Holden L, et al. Palliative radiotherapy for bone metastases in the last 3 months of life: worthwhile or futile? Clin Oncol (R Coll Radiol). 2011; 23:709–715. PMID: 21665446.

59. Poulter CA, Cosmatos D, Rubin P, Urtasun R, Cooper JS, Kuske RR, et al. A report of RTOG 8206: a phase III study of whether the addition of single dose hemibody irradiation to standard fractionated local field irradiation is more effective than local field irradiation alone in the treatment of symptomatic osseous metastases. Int J Radiat Oncol Biol Phys. 1992; 23:207–214. PMID: 1374061.

60. Jeremic B. Single fraction external beam radiation therapy in the treatment of localized metastatic bone pain. A review. J Pain Symptom Manage. 2001; 22:1048–1058. PMID: 11738168.

61. Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003; 55:594–605. PMID: 12573746.

62. Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy--a systematic review of randomised trials. Clin Oncol (R Coll Radiol). 2003; 15:345–352. PMID: 14524489.

63. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007; 25:1423–1436. PMID: 17416863.

64. Rades D, Schild SE, Abrahm JL. Treatment of painful bone metastases. Nat Rev Clin Oncol. 2010; 7:220–229. PMID: 20234353.

65. Nomiya T, Teruyama K, Wada H, Nemoto K. Time course of pain relief in patients treated with radiotherapy for cancer pain: a prospective study. Clin J Pain. 2010; 26:38–42. PMID: 20026951.

66. Huisman M, van den Bosch MA, Wijlemans JW, van Vulpen M, van der Linden YM, Verkooijen HM. Effectiveness of reirradiation for painful bone metastases: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2012; 84:8–14. PMID: 22300568.

67. Smith H, Navani A, Fishman SM. Radiopharmaceuticals for palliation of painful osseous metastases. Am J Hosp Palliat Care. 2004; 21:303–313. PMID: 15315195.

68. Roqué I Figuls M, Martinez-Zapata MJ, Scott-Brown M, Alonso-Coello P. Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev. 2011; (7):CD003347. PMID: 21735393.

69. Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med. 2010; 40:89–104. PMID: 20113678.

70. Robinson RG, Preston DF, Spicer JA, Baxter KG. Radionuclide therapy of intractable bone pain: emphasis on strontium-89. Semin Nucl Med. 1992; 22:28–32. PMID: 1589803.

71. Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol. 2005; 6:392–400. PMID: 15925817.

72. Samarium-153 lexidronam for painful bone metastases. Med Lett Drugs Ther. 39:1997; 83–84. PMID: 9286284.
73. Alberts AS, Smit BJ, Louw WK, van Rensburg AJ, van Beek A, Kritzinger V, et al. Dose response relationship and multiple dose efficacy and toxicity of samarium-153-EDTMP in metastatic cancer to bone. Radiother Oncol. 1997; 43:175–179. PMID: 9192964.

74. Sartor O, Reid RH, Bushnell DL, Quick DP, Ell PJ. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007; 109:637–643. PMID: 17167764.

75. Nazario J, Hernandez J, Tam AL. Thermal ablation of painful bone metastases. Tech Vasc Interv Radiol. 2011; 14:150–159. PMID: 21767782.

76. Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol. 2007; 10:120–131. PMID: 18070690.

77. Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L, et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol. 2011; 21:2004–2010. PMID: 21533865.

78. Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000; 175:1263–1266. PMID: 11044019.
79. Tancioni F, Lorenzetti MA, Navarria P, Pessina F, Draghi R, Pedrazzoli P, et al. Percutaneous vertebral augmentation in metastatic disease: state of the art. J Support Oncol. 2011; 9:4–10. PMID: 21465731.

80. Peh WC, Gilula LA. Percutaneous vertebroplasty: indications, contraindications, and technique. Br J Radiol. 2003; 76:69–75. PMID: 12595329.

81. Lee B, Franklin I, Lewis JS, Coombes RC, Leonard R, Gishen P, et al. The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies. Eur J Cancer. 2009; 45:1597–1602. PMID: 19223174.

82. Saliou G, Kocheida el M, Lehmann P, Depriester C, Paradot G, Le Gars D, et al. Percutaneous vertebroplasty for pain management in malignant fractures of the spine with epidural involvement. Radiology. 2010; 254:882–890. PMID: 20177099.

83. Kassamali RH, Ganeshan A, Hoey ET, Crowe PM, Douis H, Henderson J. Pain management in spinal metastases: the role of percutaneous vertebral augmentation. Ann Oncol. 2011; 22:782–786. PMID: 20966180.

84. Qian Z, Sun Z, Yang H, Gu Y, Chen K, Wu G. Kyphoplasty for the treatment of malignant vertebral compression fractures caused by metastases. J Clin Neurosci. 2011; 18:763–767. PMID: 21507652.

85. Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002; 20:4040–4049. PMID: 12351602.

86. Deer T, Krames ES, Hassenbusch SJ, Burton A, Caraway D, Dupen S, et al. Polyanalgesic consensus conference 2007: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2007; 10:300–328. PMID: 22150890.

87. Deer TR, Smith HS, Burton AW, Pope JE, Doleys DM, Levy RM, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician. 2011; 14:E283–E312. PMID: 21587338.
88. Papachristou DJ, Basdra EK, Papavassiliou AG. Bone metastases: molecular mechanisms and novel therapeutic interventions. Med Res Rev. 2012; 32:611–636. PMID: 20818675.

89. Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999; 402:304–309. PMID: 10580503.

90. Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999; 96:3540–3545. PMID: 10097072.

91. Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003; 97:887–892. PMID: 12548591.

92. Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther. 2007; 9(Suppl 1):S7. PMID: 17634146.

93. Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, et al. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain. 2010; 133:2549–2564. PMID: 20802203.

94. Chen J, Wang L, Zhang Y, Yang J. P2Y1 purinoceptor inhibition reduces extracellular signal-regulated protein kinase 1/2 phosphorylation in spinal cord and dorsal root ganglia: implications for cancer-induced bone pain. Acta Biochim Biophys Sin (Shanghai). 2012; 44:367–372. PMID: 22349022.

95. Tomura H, Mogi C, Sato K, Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal. 2005; 17:1466–1476. PMID: 16014326.

96. Huang CW, Tzeng JN, Chen YJ, Tsai WF, Chen CC, Sun WH. Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol Cell Neurosci. 2007; 36:195–210. PMID: 17720533.

97. Chen YJ, Huang CW, Lin CS, Chang WH, Sun WH. Expression and function of proton-sensing G-protein-coupled receptors in inflammatory pain. Mol Pain. 2009; 5:39. PMID: 19602228.

98. Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, Rey JM, García-García A. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat Rev. 2009; 35:707–713. PMID: 19758758.

99. Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res. 1996; 113:143–151. PMID: 9009732.
100. Sin WC, Zhang Y, Zhong W, Adhikarakunnathu S, Powers S, Hoey T, et al. G protein-coupled receptors GPR4 and TDAG8 are oncogenic and overexpressed in human cancers. Oncogene. 2004; 23:6299–6303. PMID: 15221007.

101. Hang LH, Yang JP, Yin W, Wang LN, Guo F, Ji FH, et al. Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur J Neurosci. 2012; 36:2107–2117. PMID: 22515300.

102. Lozano-Ondoua AN, Hanlon KE, Symons-Liguori AM, Largent-Milnes TM, Havelin JJ, Ferland HL 3rd, et al. Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists. J Bone Miner Res. 2013; 28:92–107. PMID: 22903605.

103. Hu S, Mao-Ying QL, Wang J, Wang ZF, Mi WL, Wang XW, et al. Lipoxins and aspirin-triggered lipoxin alleviate bone cancer pain in association with suppressing expression of spinal proinflammatory cytokines. J Neuroinflammation. 2012; 9:278. PMID: 23268791.

Fig. 4
Two different types of breakthrough pain (BTPs) and their "matching" opioid treatment. CRO: controlled release opioid, GOBTP: gradual onset breakthrough pain, IRO: immediate release opioid, ROBTP: rapid onset breakthrough pain, ROO: rapid onset opioid.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download