This article has been
cited by other articles in ScienceCentral.
Abstract
It is easy to overlook osteochondral lesions (OCLs) of the ankle in patients with chronic lower limb pain, such as complex regional pain syndrome (CRPS) or thromboangiitis obliterans (TAO, Buerger's disease). A 57-year-old woman diagnosed with type 1 CRPS, and a 58-year-old man, diagnosed with TAO, complained of tactile and cold allodynia in their lower legs. After neurolytic lumbar sympathethic ganglion block and titration of medications for neuropathic pain, each subject could walk without the aid of crutches. However, they both complained of constant pain on the left ankle during walking. Focal tenderness was noted; subsequent imaging studies revealed OCLs of her talus and his distal tibia, respectively. Immediately after percutaneous osteoplasties, the patients could walk without ankle pain. It is important to consider the presence of a hidden OCL in chronic pain patients that develop weight-bearing pain and complain of localized tenderness on the ankle.
Go to :

Keywords: ankle, cementoplasty, complex regional pain syndrome, osteochondritis dissecans, thromboangiitis obliterans
Patients with tactile allodynia resist palpation on physical examination. As a result, physicians may ignore constant weight-bearing ankle pain in patients with tactile allodynia. Patients with osteochondral lesions (OCLs) of the ankle usually complain of constant localized ankle pain from the beginning of walking, after relief of allodynia. Then, physicians can palpate the ankle and find a cause for the tenderness.
An OCL of the talus or tibia is the collective term for a focal lesion involving the talar or tibial hyaline cartilage and its underlying subchondral bone. These lesions are usually caused by single or multiple traumatic events, which lead to partial or complete detachment of a bone fragment. The lesion causes deep ankle pain associated with weight-bearing, impaired function, limited range of motion, stiffness, catching, locking, and swelling [
1,
2].
An OCL of the ankle may be obscured after a long history of diagnosis with complex regional pain syndrome (CRPS) or thromboangiitis obliterans (TAO, Buerger's disease). An OCL which causes chronic ankle pain often accompanies history of trauma to the ankle with non-traumatic ischemic conditions such CRPS or TAO.
We report 2 different cases of OCLs of the talus and tibia of the ankle in patients with different CRPS and TAO, respectively.
CASE REPORT
A 57-year-old woman developed type 1 CRPS below the ankle after a pedestrian-motor vehicle trauma involving her left foot 2 years ago. The patient complained of severe tactile and cold allodynia below the ankle, like most of patients with CRPS. The patient resisted palpation on physical examination because of severe tactile allodynia. Lumbar sympathectomy was performed to relieve cold allodynia. Neuropathic medications, including pregabalin, nortriptyline, and tramadol, were administrated in order to reduce tactile and cold allodynia. Following relief from allodynia, the patient complained of left ankle pain over 70 on a visual analogue scale [VAS: from no pain (= 0) to worst pain imaginable (= 100)] on walking within 1 minute. She showed focal tenderness on her ankle upon physical examination. Magnetic resonance imaging (MRI) revealed an OCL of the left talus (
Fig. 1A).
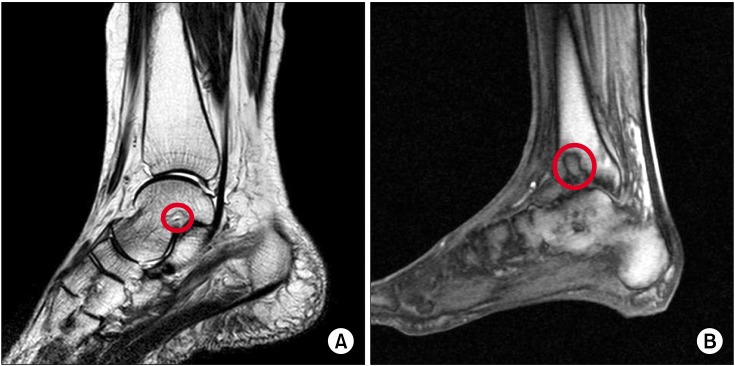 | Fig. 1Preoperative magnetic resonance images showed osteochondral lesions (red circle) of the left talus sized 11.9 × 6.0 × 15.0 mm (A) and the left tibia sized 9.4 × 17.1 × 22.5 mm (B). 
|
A 58-year-old man who suffered from TAO in both lower legs for 20 years complained of severe tactile and cold allodynia in both lower legs. The patient presented to the hospital in a wheelchair. He could walk freely without aid until 10 years ago, at which time he sprained his ankle while playing tennis. After lumbar sympathectomy and titration of neuropathic medications, the patient could walk without the aid of crutches. However, he complained of constant pain of the left ankle over 70 on a VAS on walking within 5 minutes. Physical examination revealed focal tenderness of the left ankle. Imaging studies showed an OCL of the left distal tibia (
Fig. 1B).
Both patients were scheduled for aspiration and osteoplasty with hydroxyapatite under fluoroscopic guidance. We explained the risks of the procedure to the patients before receiving informed consent. We described the risks as possible leakage of bone cement into the ankle joint, infection, bleeding, and joint stiffness or locking.
Basic non-invasive monitoring blood pressure, electrocardiography, and pulse oximetry was performed throughout the procedure. One milligram of cefazolin was infused intravenously 30 min prior to the procedure. To reduce intraoperative pain, we also intravenously injected 30 mg of ketorolac and 50 µg of fentanyl before the operation.
The patients were placed in a supine position, and the skin was prepared and draped in a sterile fashion. All procedures were performed with fluoroscopic guidance. A line representing the talocalcaneal (subtalar)/tibiotalar (ankle) joint and a circle representing the cyst were drawn on their left ankles, respectively. A 26-gauge needle was inserted to introduce local anesthetics into the skin over the cyst and ankle joint. A 13- or 11-gauge bone biopsy needle for the talus or tibia, respectively, was inserted into the cyst, and aspiration was performed. Aspirated fluid from the cyst was white and clear. After aspiration, contrast medium was injected, and the shape of the spread was observed. Bone cement comprising hydroxyapatite (Spine-Fix®, Teknimed SAS, Vic en Bigorre, France) was injected to fill the bone defect of the cyst. We injected 1.5 and 2 ml volumes of the cement into the talus and tibia, respectively. There was no cement leakage into the vessels or articular space (
Fig. 2). Postoperative computed tomography images showed that the bone cement had filled the defects (
Fig. 3).
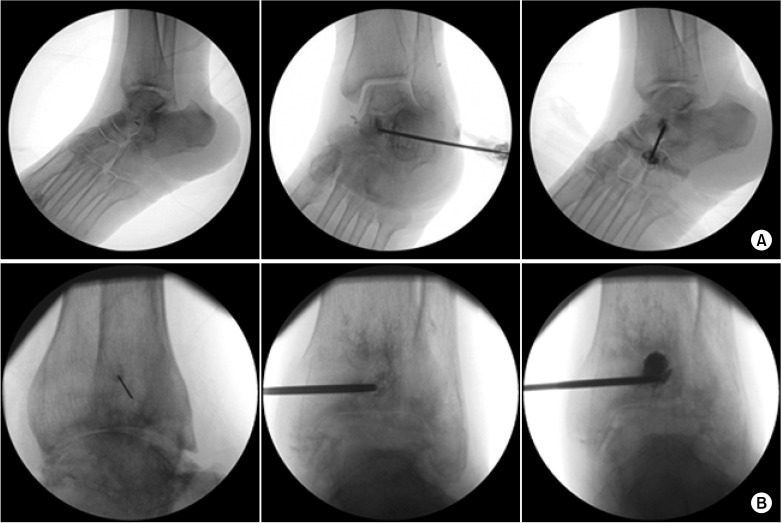 | Fig. 2Intraoperative fluoroscopic images. (Left) A 26-gauge needle was inserted to infuse local anesthetics into the skin over the cyst on the left talus (A) or tibia (B). (Middle) Contrast medium was injected into the osteochondral lesion (OCL) of the left talus (A) or tibia (B) before injection of bone cement, respectively. (Right) The OCLs were filled with bone cement. 
|
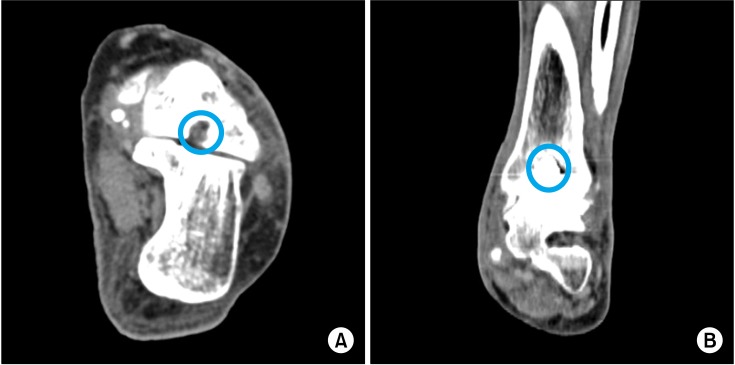 | Fig. 3Postoperative computed tomography images. The left talus (A) and tibia (B) were filled with the bone cement (blue circle). 
|
Immediately after percutaneous osteoplasties (POPs), both patients could walk without constant local ankle pain less than 30 on a VAS and experienced full of range of motion. No complications were detected at the 1 week, 1 month, 3 month, and 6 months postoperative follow-ups.
Go to :

DISCUSSION
Most OCLs of the ankle are caused by a single trauma or repeated microtrauma. Non-traumatic OCLs, in contrast, are associated with ischemia, subsequent necrosis, and perhaps genetic factors. A lesion may heal, remain asymptomatic, or progress to deep ankle pain on weight-bearing with prolonged joint swelling and the formation of subchondral bone cysts. During loading, compression of the cartilage forces water into the microfractured subchondral bone. The increased flow and pressure of fluid in the subchondral bone can cause osteolysis and trigger slow development of a subchondral cyst. The pain does not arise from the cartilage lesion but most likely is caused by repetitive high fluid pressure during walking and a concomitant decrease in pH produced by osteoclasts. These physiologic changes sensitize the highly innervated subchondral bone [
3].
There are 2 common patterns of OCLs of the talus. Anterolateral talar dome lesions result from inversion and dorsiflexion injuries of the ankle in the area in contact with the fibula. Posteromedial lesions result from inversion, plantar flexion, and external rotation injuries of the ankle in the area in contact with the tibial ceiling of the ankle joint [
4,
5].
OCLs of the tibial plafond are rare, particularly in comparison to the incidence of OCLs of the talus. This may be because of differences in the thickness and mechanical properties of the cartilage in these regions, and the rich arterial supply to the distal tibia. Distal tibial cartilage has been shown to be stiffer than talar cartilage. Anterolateral and posteromedial cartilages at the distal tibia are also stiffer than the corresponding areas on the talar dome, with the softest cartilage found in the posterior half of the talus [
6].
Several mechanisms may be responsible for the ischemia and pain in chronic CPRS. Ischemia-reperfusion injury, a possible cause of type I CRPS, produces a microvascular injury and slow-flow/no-reflow in the capillaries of the muscles and digital nerve. This slow/no reflow phenomenon initiates and maintains deep tissue ischemia and inflammation. These conditions lead to activation of muscle nociceptors, and the ectopic activation of sensory afferent axons due to endoneurial ischemia and inflammation. During the chronic stage of CRPS, impaired microcirculation is related to increased vasoconstriction, tissue hypoxia, and metabolic tissue acidosis in the affected limb [
7-
9].
TAO is a segmental, non-atherosclerotic, inflammatory disease that most commonly affects the small and medium-sized arteries, veins, and nerves of the extremities [
10]. The acute phase is composed of an occlusive, highly cellular, inflammatory thrombus. In the subacute phase, progressive organization of the thrombus takes place. The chronic phase is characterized by organized thrombus and vascular fibrosis that may mimic atherosclerotic disease [
11].
Commonly missed diagnoses in patients with chronic ankle pain include either fractures of the osteochondral talar dome, lateral talar process, calcaneal anterior process, lateral malleolar, posterolateral distal fibular flake, fifth metatarsal base, and the navicular bone or injuries of the Achilles, peroneal, posterior tibial, anterior tibial, and flexor hallucis longus tendons. If the primary problem is ankle pain, a concentrated effort should be made to rule out occult fracture of the foot or ankle. A bone scan is an excellent screening test to rule out occult fractures and to guide further treatment. If the bone scan reveals increased uptake in a discrete area, a spot radiograph or computed tomography (CT) scan is useful to further identify the exact location of the fracture. Occult or associated injuries of the tendons of the foot and ankle should also be considered, and MRI is the most useful exam to identify and confirm these injuries. Both CT and MRI are useful for the diagnosis of OCL. However, bone analysis is more precise with CT, and articular analysis can be superior with MRI [
12].
To prevent cement leakage into the joint space, it is better to place an ankle arthroscope for the direct vision of OCL, if available. However, it may increase postoperative ankle pain for a few days, need assistant's help to hold the arthroscope, and obscure the fluoroscopic view during the procedure [
12]. If there is leakage of the contrast medium into the vessel or joint, it is better to insert a slice of gelatin spongy into the needle before injecting the cement. It is not absolutely necessary to fill the maximum amount of cement because the hydroxyapatite can provide a suitable framework for osteogenesis. Injecting aid makes it easier to inject relative hard consistency of cement. If the cement flows out from the tip of the needle under lateral fluoroscopic view, injection speed has to be reduced for preventing inflow of cement into the vessels or joint space.
When symptoms persist after a 6-month trial period of conservative treatment for OCLs ≤ 15 mm in size, debridement and drilling/microfracturing is recommended. When the lesion is cystic and > 15 mm in size, cancellous bone grafting, osteochondral autograft transfer, or autologous chondrocyte implantation with cancellous bone should be considered [
2]. In the current study, preoperative MRI revealed an abnormally high signal area surrounded with marrow edema sized 11.9 × 6.0 × 15.0 mm on the talus and 9.4 × 17.1 × 22.5 mm on the tibia, respectively. Although even the sizes of the lesion were < 15 mm in diameter, bone cement insertion after aspiration of the bone cyst was considered necessary, given the continuous pain experienced upon weight-bearing. The ideal bone substitute must be easily fabricated and preserved, biocompatible, and biodegradable. Hydroxyapatite was chosen because porous and granular hydroxyapatite implanted into bony defects has been proven to provide a suitable framework for osteogenesis, and compares well with allografts and xenografts. Short- and long-term follow-up studies have shown the biological and mechanical benefits of implanted hydroxyapatite [
13,
14].
It is important to examine for the presence of a hidden OCL in patients who develop weight-bearing pain in ankle and have localized ankle tenderness under chronic lower limb ischemic conditions.
Go to :

ACKNOWLEDGEMENTS
This study was supported by a grant of a 2-year study from Pusan National University.
Go to :

References
1. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010; 18:238–246. PMID:
19859695.

2. van Bergen CJ, de Leeuw PA, van Dijk CN. Treatment of osteochondral defects of the talus. Rev Chir Orthop Reparatrice Appar Mot. 2008; 94:398–408. PMID:
19046699.

3. van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. The natural history of osteochondral lesions in the ankle. Instr Course Lect. 2010; 59:375–386. PMID:
20415393.
4. Kreuz PC, Steinwachs M, Erggelet C, Lahm A, Henle P, Niemeyer P. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med. 2006; 34:55–63. PMID:
16157849.

5. Chew KT, Tay E, Wong YS. Osteochondral lesions of the talus. Ann Acad Med Singapore. 2008; 37:63–68. PMID:
18265900.
6. O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010; 38:392–404. PMID:
19561175.
7. Coderre TJ, Bennett GJ. A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): pain due to deep-tissue microvascular pathology. Pain Med. 2010; 11:1224–1238. PMID:
20704671.

8. Groeneweg G, Huygen FJ, Coderre TJ, Zijlstra FJ. Regulation of peripheral blood flow in complex regional pain syndrome: clinical implication for symptomatic relief and pain management. BMC Musculoskelet Disord. 2009; 10:116. PMID:
19775468.

9. Wasner G. Vasomotor disturbances in complex regional pain syndrome--a review. Pain Med. 2010; 11:1267–1273. PMID:
20704675.

10. Olin JW, Shih A. Thromboangiitis obliterans (Buerger's disease). Curr Opin Rheumatol. 2006; 18:18–24. PMID:
16344615.

11. Piazza G, Creager MA. Thromboangiitis obliterans. Circulation. 2010; 121:1858–1861. PMID:
20421527.

12. Seo SS, Park JY, Kim HJ, Yoon JW, Park SH, Kim KH. Percutaneous osteoplasty for the treatment of a painful osteochondral lesion of the talus: a case report and literature review. Pain Physician. 2012; 15:E743–E748. PMID:
22996869.
13. Matsumine A, Myoui A, Kusuzaki K, Araki N, Seto M, Yoshikawa H, et al. Calcium hydroxyapatite ceramic implants in bone tumour surgery. A long-term follow-up study. J Bone Joint Surg Br. 2004; 86:719–725. PMID:
15274270.
14. Yamamoto T, Onga T, Marui T, Mizuno K. Use of hydroxyapatite to fill cavities after excision of benign bone tumours. Clinical results. J Bone Joint Surg Br. 2000; 82:1117–1120. PMID:
11132269.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download