INTRODUCTION
The C-arm fluoroscope is frequently utilized and is absolutely necessary to modern pain medicine [
1]. Medical doctors who perform C-arm fluoroscopy-guided procedures are exposed to X-rays. With the continual increase in the worldwide use of X-ray imaging, new challenges arise for the occupational radiation protection of the medical staff [
2]. The amount of radiation absorbed by an individual's tissues corresponds with the risk of developing biologic effects [
3]. Such effects are largely divided into two types. The "early effects" correspond to acute radiation lethality, local tissue damage on the skin or gonads, hematologic effects, and cytogenetic effects, while the "late effects" are radiation-induced malignancies, such as leukemia and other forms of cancer, deleterious local tissue effects, chromosomal toxicity, and/or cataract formation [
4]. The adverse effects of ionizing radiation on the human body include skin diseases, thyroid cancer, brain tumors, cataracts, and so on [
5]. The US FDA reported 26 burn complications due to fluoroscopy between 1992 and 1995 [
6]. As a result, radiation-protective methods are recommended to protect medical staff from radiation damages. Radiation-protective shields have different designs: these designs include aprons with front coverage only, aprons that wrap around the body, and two-piece garments with a vest and kilt [
7]. Protective garments are wrapped in plain clothes, with the radiation-protective materials inside [
8]. Thus, even if there is no damage to the outer portion of the apron, we cannot guarantee the apron's functionality because there is the possibility that the inner material may be damaged. In general, shields must be used whenever possible to lower the radiation exposure level to a reasonably achievable limit, while not lengthening the fluoroscopy-guided procedure and compromising the patient's safety [
7]. Although radiation-protective shields and thyroid protectors are essential equipment in reducing the radiation exposure of medical doctors who perform interventional procedures, they often have been used for several years without regular inspection. The aim of this study was to investigate the degree of damage to radiation-protective shields in the operating room.
Go to :

MATERIALS AND METHODS
This study investigated 98 radiation-protective shields (aprons and thyroid protectors) in the operation rooms of Konkuk University Medical Center (KUMC) and Jeju National University Hospital (JNUH). We examined whether these protective shields were externally damaged or not with the unaided eye. Also, we checked for any signs of defects, such as holes, cracks, separations and/or pieces which were collapsed down by C-arm fluoroscope. If the radiation-protective shields had cracks or holes, we recorded them and sorted them out by the introduction year at the hospital and location of the damage. For one month, we observed whether doctors and nurses in the operation rooms that use C-arm fluoroscopes dressed in aprons and thyroid shields or not.
We examined the degree to which radiation-protective shields were utilized in each hospital; their utilization by pain clinicians (professors/fellows, residents, and nurses) and surgery personnel was compared with a chi-square test using the SPSS software (version 19.0, SPSS, Inc., USA). Statistical significance was defined as P < 0.05.
Go to :

RESULTS
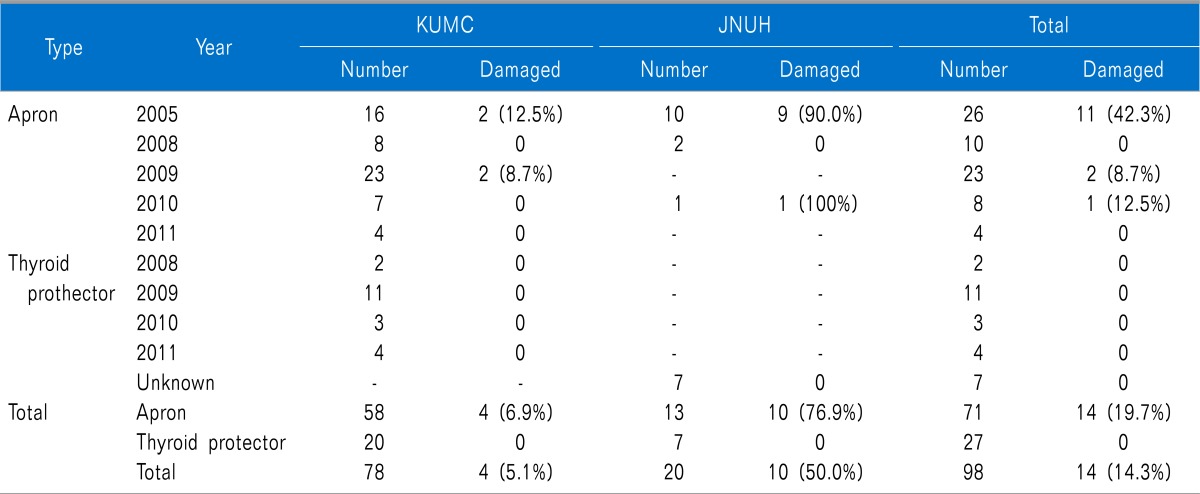
There were seventy-one aprons and twenty-seven thyroid protectors examined for this study. At KUMC, there were fifty-eight aprons and twenty thyroid protectors. At JNUH, there were thirteen aprons and seven thyroid protectors. When examined with the naked eye, there was no apparent damage to the radiation-protective shields. However, defects in the shields were found using fluoroscopy (
Fig. 1). Fourteen aprons (19.7%) were damaged, whereas all thyroid shields were normal. The damage to the aprons was classified as holes, cracks, separations, and collapses. Nine holes, seven cracks, five separations, and four collapses were found. Some aprons showed combinations of defects. Of the twenty-six aprons, which have been used since 2005, eleven (42.3%) of them were damaged. Of the ten aprons that have been used since 2008, none (0%) of them were damaged. Of the twenty-three aprons that have been used since 2009, two (8.7%) were damaged. Of the eight aprons that have been used since 2010, one (12.3%) was damaged. Of the four aprons used since 2011, none (0%) of them were damaged. At KUMC, four aprons (6.9%) were damaged, while at JNUH, ten aprons (76.9%) were damaged. The older products, which have been used since 2005, had the highest percentage of damage to the aprons (
Table 1). The most common site of damage to the radiation-protective shields was the waist of the apron (51%). The second most common site of damage was the lower part of the apron (33%) (
Fig. 2).
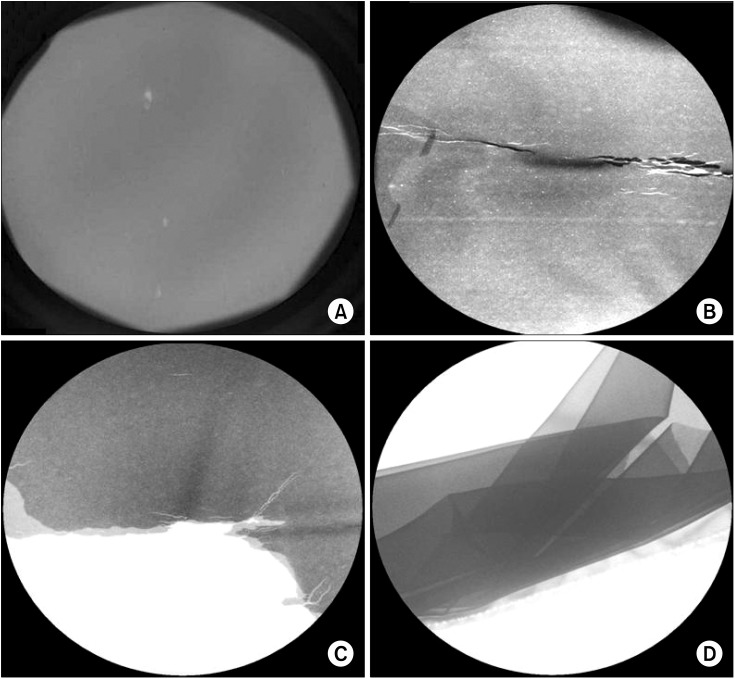 | Fig. 1C-arm fluoroscopic images of radiation-protective shields. (A) Hole. (B) Cracked. (C) Separated. (D) Collapsed down. 
|
 | Fig. 2Damage site and the rate. The most common damaged site of radiation-protective shields was waist of aprons (40%). (A) One-piece type apron. (B) Kilt of two piece type apron. 
|
Table 1
Data Ofradiation-protective Shields

Thyroid protectors were utilized to a significantly lower extent than aprons at KUMC and JNUH and were less utilized during surgery than during pain management. In professors/fellows, the degree of utilization of aprons used by surgery personnel was significantly lower than that of pain physicians. The degree of utilization of thyroid protectors by surgery personnel was significantly lower than that of the pain clinicians. Almost all pain clinicians wore thyroid shields during procedures, but fewer than half of the surgery personnel wore them (
Fig. 3).
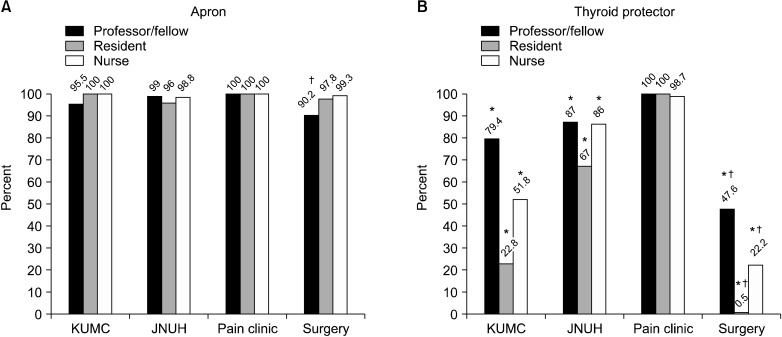 | Fig. 3Degree of utilization of radiation-protective shields. (A) Apron. (B) Thyroid protector. KUMC: Konkuk University Medical Center, JNUH: Jeju National University Hospital. *P < 0.05 Apron versus Thyroid protector. †P < 0.05 Pain clinic versus Surgery. 
|
Go to :

DISCUSSION
At KUMC, the rate of damage to the aprons that had been introduced in 2005 was the highest because physicians had used them for three years without receiving any new supplies. There were no damages found in aprons which were introduced after 2008, and only two aprons which were introduced in 2009 found to be damaged. An increase in the number of aprons might result in a decrease in the frequency of use for a single apron. At JNUH, the overall rate of damage to the aprons was 90%. The total number of aprons at JNUH was thirteen, among them ten aprons were introduced in 2005. As a result, a longer period and more frequent use of the same protector was thought to be a possibility for the increase in damage. The most common sites of damage to the aprons were at the waist. This is probably due to waistbands causing tension on the aprons' waist sections, which are frequently folded while walking and sitting (
Fig. 4). There were four two-piece garments at KUMC. There were two damaged kilts whereas there was no damaged vest (
Fig. 2B). Male residents (surgeons) tend to wear kilts without vests at KUMC therefore kilts were used more frequently than vests. Aprons have more folds, so can break easily. As a result, aprons should be stored on a hanger in order to minimize folds which can lead to damage. In addition, aprons should be inspected fluoroscopically on an annual basis to check for holes and a reduction in shielding integrity [
7].
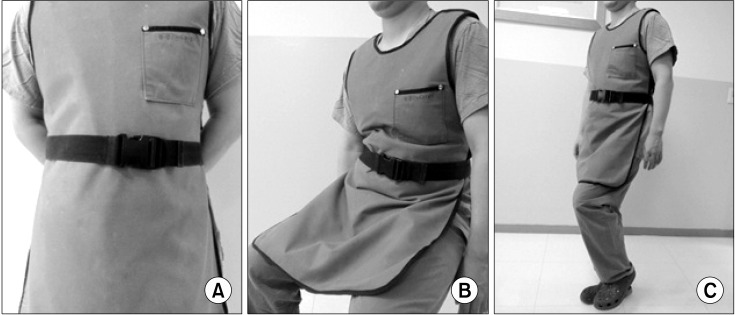 | Fig. 4The folding sites of apron according to physician's position. (A) Standing. (B) Sitting. (C) Walking. 
|
In this study, the degree of utilization of radiation-protective shields for surgeons was significantly lower than that of pain physicians. This is thought to be due to the low awareness or knowledge of radiation safety. In addition, this result seems to be related to the surgeon wearing an aseptic gown over the radiation-protective shields. In these cases, the doctor may feel excessive heat and discomfort. Although the number of thyroid shields is less than that of aprons, there has been no lack of thyroid shields for clinicians to use. Therefore, the lack of damage to the thyroid protectors may not be related to the lower number of thyroid shields, but instead may be related to the lower utilization of thyroid shields than that of aprons.
The amount of radiation exposure is proportional to the duration of exposure, while being inversely proportional to the square of the distance from the radiation source [
9]. This is also proportional to the damaged areas of the aprons. If shielding materials collapses inside the apron, the apron cannot protect the wearer from radiation exposure. Radiation exposure results from the primary beam and the scatter radiation reflected off the floor and the patient [
10]. The practitioner receives the highest dosage overall in comparison to the assistants and scrub nurses [
11]. Standing 1 m away before obtaining an image can significantly reduce the levels of radiation exposure to the staff members [
1]. In most areas, radiation-protective shields require a thickness of at least 0.5 mm of lead-equivalents to be used, which attenuates over 90% of the scattered x-rays that strike it [
7]. According to another study, in C-arm fluoroscopy-guided procedures, exposure in terms of mREM should be 2,690 mREM outside the apron with 0 mREM inside the apron, whereas it should be 4.03 mREM outside the apron for the entire body (head and trunk) with an effective dose equivalent exposure of 0 mREM per year inside the apron [
12].
The International Commission on Radiological Protection (ICRP) has set an annual maximum permissible radiation dose to reduce damages caused by radiation exposure, with the ICRP strongly suggesting that radiation exposure be within these set limits [
13]. The annual allowance of radiation exposure is 50 mSv. In Europe, however, exposure should not exceed 20 mSv/year on average over 5 years; in the U.S., regulations limit exposure to only 10 mSv/year over a person's entire life [
14].
The cardinal principles of radiation protection are: (1) maximize the distance from the radiation source; (2) use shielding materials; and (3) minimize exposure time [
10]. Precautions should be taken to reduce exposures as much as possible. Potential decreases in radiation exposure can be accomplished by decreased exposure time; increased distance; increased shielding with aprons, thyroid gland covers, gloves, and glasses; beam collimation; using the low-dose option available on some C-arm units; inverting the C-arm; and surgeon control of the C-arm [
15].
Available patient dose reduction technologies include low fluoroscopy dose rate settings, low frame rate pulsed fluoroscopy, spectral beam filtration, and the use of increased X-ray beam energy [
14]. Application of collimation can exclude areas of greatly varying radiodensity to improve the image quality by reducing the range of densities included in the field [
16]. Using the pulsed mode and low-dose modes together showed a significant decrease in the dose of radiation absorbed than in any other mode [
17].
In this investigation, the shielding material in protective garments can develop cracks and holes over time, although they may not be visible externally. To help prevent this, aprons should be stored on hangers with minimum folding. Also, garments should be monitored annually with fluoroscopy to check for holes and reduced shielding integrity [
7]. To make fluoroscopy use safer, education about, attention to, and practices of radiation safety should be required for all pain physicians [
18].
This study had some limitations. We did not investigate the frequency of use of each radiation protector, but only the periods of usage of the radiation protectors. It was impossible to assess how many times each protector had been used. Therefore, we supposed that the period was related to the frequency of use. In this study, only two hospitals were involved. If more institutes were included, the data might be better than our current findings.
In conclusion, radiation-protective shields, which have been used for a long time, can have a higher risk of damage. Radiation-protective shields should be inspected regularly and exchanged for new products for the radiation safety of medical workers.
Go to :


