1. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004; 4:253–265. PMID:
15057285.

2. Forsyth PA, Balmaceda C, Peterson K, Seidman AD, Brasher P, DeAngelis LM. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neurooncol. 1997; 35:47–53. PMID:
9266440.
3. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995; 6:489–494. PMID:
7669713.

4. Cavaletti G, Bogliun G, Marzorati L, Zincone A, Marzola M, Colombo N, et al. Peripheral neurotoxicity of taxol in patients previously treated with cisplatin. Cancer. 1995; 75:1141–1150. PMID:
7850713.

5. Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011; 152:308–313. PMID:
21145656.

6. Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006; 201:507–514. PMID:
16797537.

7. Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008; 214:276–284. PMID:
18809400.

8. Scuteri A, Nicolini G, Miloso M, Bossi M, Cavaletti G, Windebank AJ, et al. Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Res. 2006; 26:1065–1070. PMID:
16619507.
9. Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007; 1168:46–59. PMID:
17698044.

10. Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001; 429:23–37. PMID:
11698024.

11. Sekiguchi M, Sekiguchi Y, Konno S, Kobayashi H, Homma Y, Kikuchi S. Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression. Eur Spine J. 2009; 18:1978–1985. PMID:
19543754.

12. Kim SH, Nam JS, Choi DK, Koh WW, Suh JH, Song JG, et al. Tumor necrosis factor-alpha and apoptosis following spinal nerve ligation injury in rats. Korean J Pain. 2011; 24:185–190. PMID:
22220239.

13. Yu YM, Kim JB, Lee KW, Kim SY, Han PL, Lee JK. Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 2005; 36:2238–2243. PMID:
16141417.

14. Wang Q, Ding Q, Zhou Y, Gou X, Hou L, Chen S, et al. Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology. 2009; 110:1279–1286. PMID:
19417608.

15. Genovese T, Esposito E, Mazzon E, Di Paola R, Meli R, Caminiti R, et al. Beneficial effects of ethyl pyruvate in a mouse model of spinal cord injury. Shock. 2009; 32:217–227. PMID:
18948848.

16. Guo J, Zhang K, Ji Y, Jiang X, Zuo S. Effects of ethyl pyruvate on myocardial apoptosis and expression of Bcl-2 and Bax proteins after ischemia-reperfusion in rats. J Huazhong Univ Sci Technolog Med Sci. 2008; 28:281–283. PMID:
18563323.

17. Tsung A, Kaizu T, Nakao A, Shao L, Bucher B, Fink MP, et al. Ethyl pyruvate ameliorates liver ischemia-reperfusion injury by decreasing hepatic necrosis and apoptosis. Transplantation. 2005; 79:196–204. PMID:
15665768.

18. Choi DK, Leem JG, Shin JW, Suh JH. Effects of ethyl pyruvate on allodynia, TNF-α expression, and apoptosis in the dorsal root ganglion after spinal nerve ligation injury. Korean J Pain. 2012; 25:213–220. PMID:
23091681.

19. Lee MJ, Jang M, Jung HS, Kim SH, Cho IH. Ethyl pyruvate attenuates formalin-induced inflammatory nociception by inhibiting neuronal ERK phosphorylation. Mol Pain. 2012; 8:40. PMID:
22640699.

20. Jacobs CC, Holcombe SJ, Cook VL, Gandy JC, Hauptman JG, Sordillo LM. Ethyl pyruvate diminishes the inflammatory response to lipopolysaccharide infusion in horses. Equine Vet J. 2012; [in press].

21. Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther. 2006; 318:735–740. PMID:
16687474.

22. Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001; 94:293–304. PMID:
11731066.

23. Choi JS, Lee MS, Jeong JW. Ethyl pyruvate has a neuroprotective effect through activation of extracellular signal-regulated kinase in Parkinson's disease model. Biochem Biophys Res Commun. 2010; 394:854–858. PMID:
20307492.

24. Yang R, Shaufl AL, Killeen ME, Fink MP. Ethyl pyruvate ameliorates liver injury secondary to severe acute pancreatitis. J Surg Res. 2009; 153:302–309. PMID:
19027919.

25. Li Y, Dorsi MJ, Meyer RA, Belzberg AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000; 85:493–502. PMID:
10781924.

26. Choi JI, Kim WM, Yoon MH, Lee HG. Antiallodynic effect of thalidomide and morphine on rat spinal nerve ligation-induced neuropathic pain. Korean J Pain. 2010; 23:172–178. PMID:
20830262.

27. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID:
7990513.

28. Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006; 122:245–257. PMID:
16530964.

29. Pascual D, Goicoechea C, Suardíaz M, Martín MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005; 118:23–34. PMID:
16213089.

30. Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008; 135:262–270. PMID:
17659836.

31. Zhang J, Tuckett RP. Comparison of paclitaxel and cisplatin effects on the slowly adapting type I mechanoreceptor. Brain Res. 2008; 1214:50–57. PMID:
18466884.

32. Figueroa-Masot XA, Hetman M, Higgins MJ, Kokot N, Xia Z. Taxol induces apoptosis in cortical neurons by a mechanism independent of Bcl-2 phosphorylation. J Neurosci. 2001; 21:4657–4667. PMID:
11425893.

33. Jang HJ, Hwang S, Cho KY, Kim do K, Chay KO, Kim JK. Taxol induces oxidative neuronal cell death by enhancing the activity of NADPH oxidase in mouse cortical cultures. Neurosci Lett. 2008; 443:17–22. PMID:
18672029.

34. Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002; 7:313–319. PMID:
12101390.
35. Joseph EK, Levine JD. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur J Neurosci. 2004; 20:2896–2902. PMID:
15579143.

36. Burkhart CA, Berman JW, Swindell CS, Horwitz SB. Relationship between the structure of taxol and other taxanes on induction of tumor necrosis factor-alpha gene expression and cytotoxicity. Cancer Res. 1994; 54:5779–5782. PMID:
7954398.
37. Manthey CL, Brandes ME, Perera PY, Vogel SN. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992; 149:2459–2465. PMID:
1356126.
38. Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012; 12:55–61. PMID:
22019566.

39. Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012; 59:3–9. PMID:
22537849.

40. Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 2012; 13:293–303. PMID:
22285612.

41. Burgos E, Gómez-Nicola D, Pascual D, Martín MI, Nieto-Sampedro M, Goicoechea C. Cannabinoid agonist WIN 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur J Pharmacol. 2012; 682:62–72. PMID:
22374260.

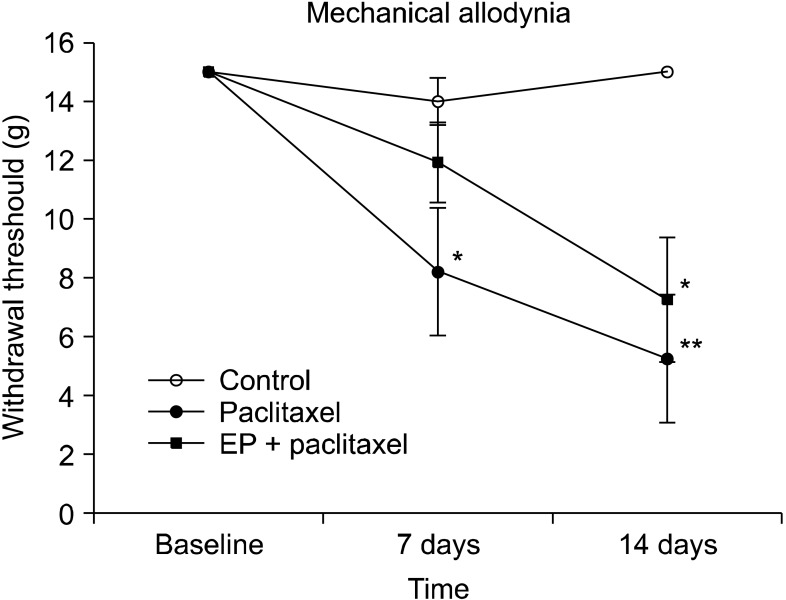
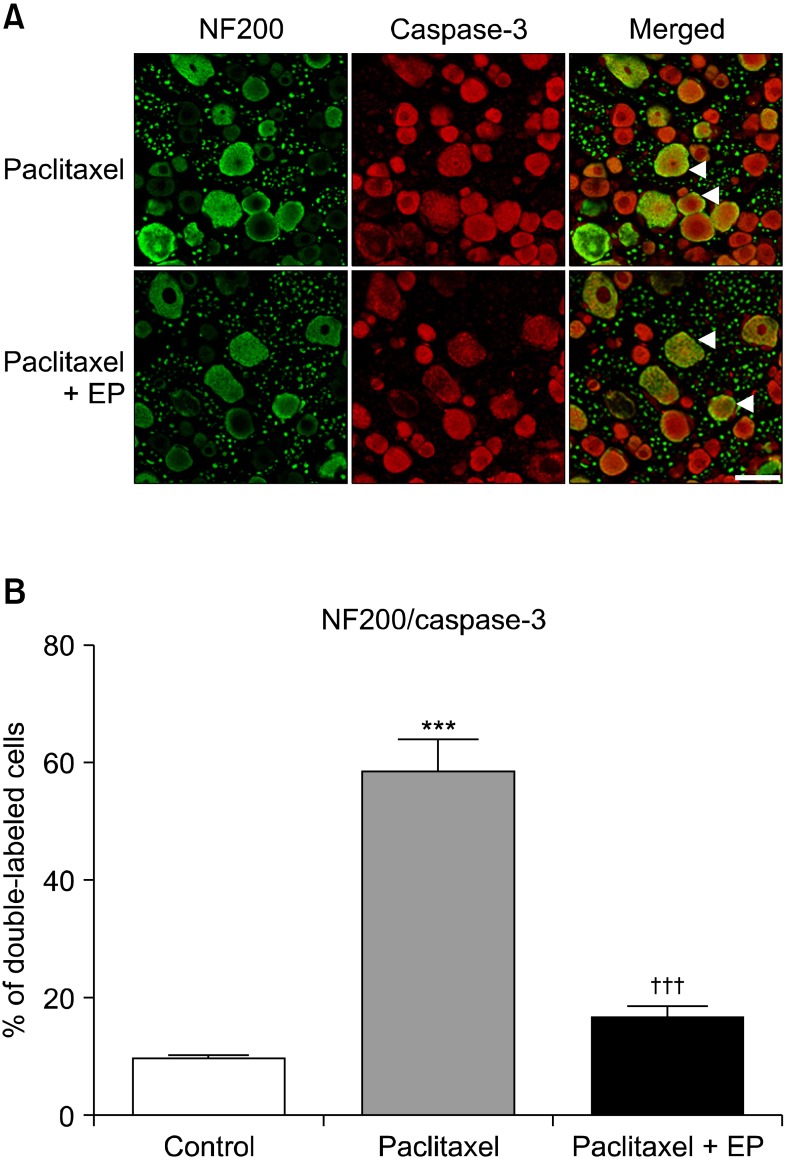
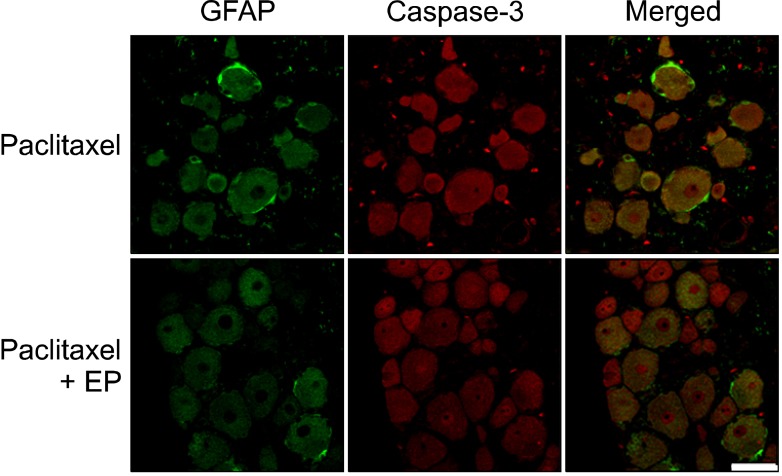




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download