Abstract
Background
5-hydroxytryptamine 3 (5-HT3) receptors have been known to be associated with the modulation of nociceptive transmission. However, it is uncertain whether 5-HT3 plays a role in the antinociceptive or pronociceptive pathway for incisional pain. In this study, we evaluated the effects of palonosetron, a 5-HT3 receptor antagonist, on incisional pain in rats when administered intrathecally or intraplantarly.
Methods
An intrathecal catheter was implanted through the cisterna magna and placed in the intrathecal space of rats. An incision in the plantaris muscle of the right hind paw was done under anesthesia with sevoflurane. Withdrawal thresholds were evaluated with the von Frey filament after 2 hours. Palonosetron (0.5 and 0.1 µg intrathecally; 0.5 µg intraplantarly) was administered and the thresholds were observed for 4 hours.
Go to : 
5-Hydroxytryptamine (5-HT) receptors are known to be located in the central and peripheral nervous system and have a role in modulating transmitted nociceptive stimulations [1]. However, 5-HT receptors play a vague role in the transmission and modulation of pain. There have been many researches which have reported the contradicting results [1-4]. 5-HT3 receptors are also located in the peripheral and central system, and their role in pain modulation remains unclear. They are located not only in nociceptive fibers of the dorsal horn but also in the descending inhibitory system, peripheral nerve, brain, and autonomic afferent fibers [1]. Stimulation of 5-HT3 receptors in the spinal cord has an antinociceptive effect on acute pain evoked by a formalin stimulus [2]. On the contrary, blocking 5-HT3 receptors showed antinociceptive effects on thermal stimuli [3]. McCleane et al. have reported that a single intravenous injection of ondansetron, a 5-HT3 receptor antagonist, significantly reduced the pain of patients with chronic neuropathic pain [4]. Therefore, we hypothesized that palonosetron, an effective 5-HT3 receptor antagonist, also could possibly modulate the transmission of pain. However, most studies have focused on neuropathic pain and there have been no studies on the effect of the 5-HT3 receptor antagonist on incisional pain. In this study, we investigated the behavioral change of rats to mechanical stimuli on incised paws before and after the administration of palonosetron intrathecally or intraplantarly, to evaluate whether palonosetron has a pronociceptive or antinociceptive effect.
Go to : 
This study was approved by the Institutional Animal Care Committee, Research Institute of Medical Science. Adult male Sprague-Dawley rats weighing 200-250 g were used for the experiment. All rats raised in cages were kept at ideal conditions with food and water available ad libitum with a 12 h night and day cycle. The rats were anesthetized with sevoflurane and intrathecal catheters were implanted through the cisterna magna for drug administration [5]. For insertion of the catheter, the atlanto-occipital membrane was incised after sterile dressing, and the catheter was advanced caudally by 8.5 cm and placed on the lumbar enlargement. After implantation of the catheter, the rats were kept in individual cages and observed for signs of neurological dysfunction. Rats that showed neurological dysfunction such as limping or sensory deficits were sacrificed immediately, and rats without neurologic deficits were sent back to the vivarium.
Five days after intrathecal catheterization, the incisional pain model by Brennan et al. [6] was applied to the prepared rats. The rats were anesthetized with sevoflurane and the plantar surface of the left hind paws were prepared for incision. After sterilization, 1 cm longitudinal incisions starting 0.5 cm from the proximal edge of heel were made and extended towards the toes. Skins and fasciae were incised to expose the plantaris muscles, and then, the muscles were elevated and incised longitudinally. Gentle pressures were applied to control bleeding and skin closures were done with 4-O silk sutures. After dressing with povidone-iodine solution, the rats were sent back to the cages to recover.
The mechanical hyperalgesia was assessed by measuring the paw withdrawal threshold in response to mechanical stimulation using von Frey filaments. An initial test for pain behavior was done before surgery and a baseline for the post-incisional threshold was evaluated 2 hours after the surgery. Only rats with marked allodynia (withdrawal threshold < 5 g) after surgery were used for the study.
Palonosetron (Aloxi®) was used for the study. Palonosetron was administered intrathecally with a gear-operated syringe pump and intraplantarly using a 0.5 ml syringe with a 28G needle. Drugs were prepared as a solution in a volume of 10 µl and were delivered intrathecally (0.5 µg or 0.1 µg) or intraplantarly (0.5 µg). The paw withdrawal threshold was measured 30, 60, 120, and 240 min after delivery of the drug. After the experiments, the rats were sacrificed with an overdose of inhalation anesthetics.
All data are expressed as the mean ± standard deviation. Behavioral experiments were analyzed by one-way ANOVA and post hoc with Scheffe test. Values with a P < 0.05 were considered statistically significant compared to the saline-treated controls.
Go to : 
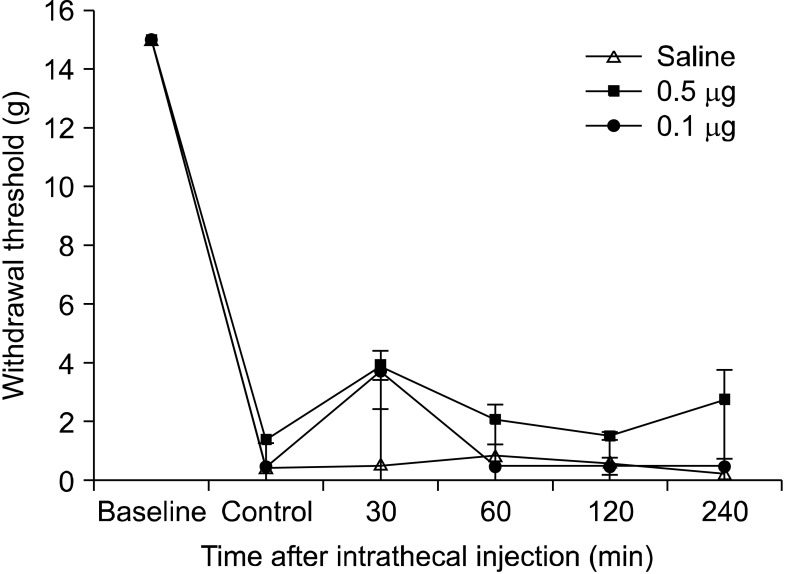
Incision of the plantar surface of the rat hind paws reduced the withdrawal threshold to mechanical stimulation significantly, and mechanical hyperalgesia was produced steadily. There were no changes in the paw withdrawal threshold during stimulation of the contralateral paw for any of the tests. When palonosetron 0.5 µg was administered intrathecally, the paw withdrawal threshold increased slightly compared to the control response with saline (Fig. 1). Thirty minutes after intrathecal administration of palonosetron (0.5 µg and 0.1 µg), the paw withdrawal threshold was higher (3.9 ± 1.9 g and 3.7 ± 0.5 g, respectively) than that of the control (0.4 ± 0.1 g) (Fig. 1). However, statistical analysis showed that neither 0.1 µg nor 0.5 µg of intrathecal palonosetron could produce a significant change compared to the control. Intraplantar injection of palonosetron (0.5 µg) also did not change the paw withdrawal threshold significantly compared to the control (Fig. 2).
Go to : 
In this study, we observed the effect of palonosetron administered intrathecally or intraplantarly on incisional pain of rats. However, palonosetron did not alter the paw withdrawal threshold.
Peripheral tissue injury during surgery activates nociceptive receptors of the peripheral and central nervous system and leads to peripheral and central sensitization [7]. This change causes hyperalgesia and allodynia of postoperative pain. Controlling postoperative pain is important in increasing the quality of life and preventing organ dysfunction or a decline in psychological status. Thus, it is important to discover drugs that decrease postoperative pain without any complications [7]. Palonosetron is a second-generation 5-HT3 receptor antagonist with relatively high-binding affinity for the 5-HT3 receptor [8]. It is frequently used to prevent postoperative nausea and vomiting (PONV) [9]. Therefore, we thought palonosetron, used to prevent PONV, could have an effect on postoperative pain if the 5-HT3 receptor antagonist modulates nociceptive transmission [1-4].
According to the literature, the 5-HT3 receptor has antinociceptive activity in acute pain models [1]. This effect is thought to be associated with the release of GABA and the activation of the descending inhibitory pathway, and the administration of a 5-HT receptor antagonist has been shown to block the antinociceptive effect [1,10]. On the other hand, the 5-HT3 receptor also has pronociceptive effect [1]. The pronociceptive effect of the 5-HT3 receptor seems to be related to the spino-bulbo-spinal loop that is activated by superficial neurokinin 1 (NK1) receptor-expressing neurons [1,11,12]. The spino-bulbo-spinal loop drives the excitatory impulse from the brainstem [11]. NK1-expressing neurons within lamina I/III in the spinal cord are associated with supraspinal targets, and they regulate the sensitivity of pain after injury [11-13]. Such neurons are projected to the parabrachial area, which is connected to the part of the brainstem that projects back down to the spinal cord [4,11,12]. These descending pathways are mediated by the activation of 5-HT3 receptors and enhance central spinal pain [4,12,13]. In studies, ablation of NK1-expressing neurons produced changes in the neurons of the dorsal horn and in pain sensation [12,13]. Studies have also shown that the pharmacological blocking of the descending serotonergic facilitatory pathway with the 5-HT3 receptor antagonist reproduced systemic changes that resembled the effect associated with ablation of NK1-expressing neurons [11,12].
At the beginning of the study, we expected that intrathecal palonosetron could also block NK1 receptor-expressing neurons and could show an antinociceptive effect. In fact, ondansetron has been shown in previous studies to significantly attenuate mechanical allodynia in an animal model [14] and decrease neuropathic pain in humans [4]. However, in this study, palonosetron did not increase the paw withdrawal threshold significantly. Although intrathecal palonosetron slightly increased the paw withdrawal threshold, an effect that was maximal at 30 minutes after injection, this increase was not statistically significant. Furthermore, palonosetron administered intraplantarly did not produce any effect at 0.5 µg which was the largest dose that we could use. Thus, 0.1 µg of palonosetron was not used for the intraplantar study.
Therefore, we conclude that 5-HT3 receptor antagonist is not effective in reducing incisional pain. We thought of some possible explanations. First, blocking the 5-HT3 receptor with palonosetron may not be effective in preventing primary hyperalgesia after incision because the action of the 5-HT3 receptor is mainly associated with the descending excitatory pathway. Spinal nociception is facilitated by the activation of the 5-HT3 receptors by 5-HT released from descending neurons. This condition contributes to central sensitization through wide dynamic range neurons, which are located at lamina V, and seems to be related to secondary hyperalgesia after incision sensitization [15]. In this study, however, we evaluated primary hyperalgesia, which is related to the sensitization of primary afferent nociceptors after incision [16]. Second, mechanisms for primary hyperalgesia after skin incision may be discordant with that of the antinociceptive effect produced by 5-HT3 receptors. Pain induced by incision may have different mechanisms from inflammatory or neuropathic pain. Pogatzki et al. has reported that rostral medial medulla does not contribute to secondary hyperalgesia after incision in rats which is dissimilar to inflammatory and neuropathic pain. Thus, incision-induced pain may be related with a different mechanism [17]. In fact, the facilitatory role of spinal 5-HT3 receptor was mostly reported in inflammatory or neuropathic states which are related to central sensitization [4,14,18,19]. Furthermore, many other receptors such as excitatory amino acid receptors and N-methyl-D-aspartate (NMDA) and non-NMDA receptors play an important role in the development of postoperative pain [16]. Additionally, a study on serotonin receptors in dorsal root ganglion neurons showed 5-HT alone had no major effect [20]. Moreover, the proton-induced current increased when 5-HT was combined with other inflammatory mediators. Thus, 5-HT alone could have little effect on incisional pain.
Serotonin receptors are also present in the peripheral nervous system and 5-HT has an excitatory effect on peripheral nerve fibers [21]. Eschalier et al. studied the effect of 5-HT3 antagonist on carrageenan-induced rat paw inflammation [22]. The 5-HT3 antagonist prevented the hyperalgesia induced by carrageenan when administered 20 min before carrageenan injection, and the effect lasted for about 90 min; however, the 5-HT3 antagonist had no preventive effect on carrageenan-induced hyperalgesia when administered 2 hours after carrageenan injection [22]. The authors concluded that 5-HT3 released by injury produce early inflammatory sensitization of the peripheral nociceptors. Therefore, it is thought that palonosetron administered 2 hours after incision could not prevent early sensitization and had no effect on the withdrawal threshold.
There are some limitations to this study. The absence of an immunoreactivity study or measurement of antagonism or synergism with other drugs is the first limitation. The second limitation concerns the doses of palonosetron used. The largest dose of palonosetron in this study was 0.5 µg. A concentration of 0.5 µg/10 µl was the maximum dose that could be obtained from the original solution. Thus, we could not use higher dosages. However, the dosage of palonosetron in this study may not be insufficient for the following reason. Palonosetron is more potent than ondansetron because of its high-binding affinity for the 5-HT3 receptor [8], therefore a smaller dose is used for PONV. Palonosetron 0.075 mg has a similar effectiveness with ondansetron 8 mg in preventing PONV [23]. Additionally, the clinical dose of ondansetron is 107 times higher than that of palonosetron. In an animal study, ondansetron 20 µg showed effects [8]. Thus, 0.19 µg of palonosetron may have a similar effectiveness with ondansetron. The maximum dose in this study (0.5 µg) was higher than 0.19 µg. Another limitation was that we did not investigate the preemptive effect of palonosetron. Administration of palonosetron before incision could have shown different results.
In conclusion, palonosetron did not show any effect on the modulation of mechanical hypersensitivity in the incisional pain model of rats when administered 0.5 µg intrathecally or intraplantarly. Further studies are needed to evaluate the effects of palonosetron using a more concentrated dose or when administered preoperatively.
Go to : 
ACKNOWLEDGEMENTS
The present study was supported by grants from the Clinical Medicine Research Institute at Chosun University Hospital, 2012.
Go to : 
References
1. Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research--evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007; 560:1–8. PMID: 17316606.

2. Jeong CY, Bae HB, Park HC, Choi JI, Yoon MH. Evaluation of the role of 5-hydroxytryptamine receptor subtypes in the regulation of nociceptive transmission in the rat spinal cord. Korean J Anesthesiol. 2004; 47:856–861.

3. Ali Z, Wu G, Kozlov A, Barasi S. The role of 5HT3 in nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Neurosci Lett. 1996; 208:203–207. PMID: 8733305.

4. McCleane GJ, Suzuki R, Dickenson AH. Does a single intravenous injection of the 5HT3 receptor antagonist ondansetron have an analgesic effect in neuropathic pain? A double-blinded, placebo-controlled cross-over study. Anesth Analg. 2003; 97:1474–1478. PMID: 14570668.

5. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036. PMID: 14677603.

6. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996; 64:493–501. PMID: 8783314.

7. Shin DJ, Yoon MH, Lee HG, Kim WM, Park BY, Kim YO, et al. The effect of treatment with intrathecal ginsenosides in a rat model of postoperative pain. Korean J Pain. 2007; 20:100–105.

8. Wong EH, Clark R, Leung E, Loury D, Bonhaus DW, Jakeman L, et al. The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol. 1995; 114:851–859. PMID: 7773546.

9. Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. Palonosetron 04-07 Study Group. A randomized, doubleblind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008; 107:439–444. PMID: 18633021.

10. Alhaider AA, Lei SZ, Wilcox GL. Spinal 5-HT3 receptor-mediated antinociception: possible release of GABA. J Neurosci. 1991; 11:1881–1888. PMID: 2066767.

11. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004; 25:613–617. PMID: 15530638.

12. Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002; 5:1319–1326. PMID: 12402039.

13. Rahman W, Suzuki R, Hunt SP, Dickenson AH. Selective ablation of dorsal horn NK1 expressing cells reveals a modulation of spinal alpha2-adrenergic inhibition of dorsal horn neurones. Neuropharmacology. 2008; 54:1208–1214. PMID: 18462764.

14. Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004; 110:259–268. PMID: 15275776.

15. Vandermeulen EP, Brennan TJ. Alterations in ascending dorsal horn neurons by a surgical incision in the rat foot. Anesthesiology. 2000; 93:1294–1302. PMID: 11046219.

16. Zahn PK, Pogatzki EM, Brennan TJ. Mechanisms for pain caused by incisions. Reg Anesth Pain Med. 2002; 27:514–516. PMID: 12373702.

17. Pogatzki EM, Urban MO, Brennan TJ, Gebhart GF. Role of the rostral medial medulla in the development of primary and secondary hyperalgesia after incision in the rat. Anesthesiology. 2002; 96:1153–1160. PMID: 11981156.

18. Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002; 22:1010–1019. PMID: 11826129.

19. Rahman W, Suzuki R, Webber M, Hunt SP, Dickenson AH. Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain. 2006; 123:264–274. PMID: 16644129.

20. Kress M, Reeh PW, Vyklicky L. An interaction of inflammatory mediators and protons in small diameter dorsal root ganglion neurons of the rat. Neurosci Lett. 1997; 224:37–40. PMID: 9132685.

21. Sommer C. Is serotonin hyperalgesic or analgesic? Curr Pain Headache Rep. 2006; 10:101–106. PMID: 16539862.

22. Eschalier A, Kayser V, Guilbaud G. Influence of a specific 5-HT3 antagonist on carrageenan-induced hyperalgesia in rats. Pain. 1989; 36:249–255. PMID: 2919105.

23. Park SK, Cho EJ. A randomized, double-blind trial of palonosetron compared with ondansetron in preventing postoperative nausea and vomiting after gynaecological laparoscopic surgery. J Int Med Res. 2011; 39:399–407. PMID: 21672343.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download