Abstract
Background
An intravenous infusion of lidocaine has been used on numerous occasions to produce analgesia in neuropathic pain. In the cases of failed back surgery syndrom, the pain generated as result of abnormal impulse from the dorsal root ganglion and spinal cord, for instance as a result of nerve injury may be particularly sensitive to lidocaine. The aim of the present study was to identify the effects of IV lidocaine on neuropathic pain items of FBSS.
Methods
The study was a randomized, prospective, double-blinded, crossover study involving eighteen patients with failed back surgery syndrome. The treatments were: 0.9% normal saline, lidocaine 1 mg/kg in 500 ml normal saline, and lidocaine 5 mg/kg in 500 ml normal saline over 60 minutes. The patients underwent infusions on three different appointments, at least two weeks apart. Thus all patients received all 3 treatments. Pain measurement was taken by visual analogue scale (VAS), and neuropathic pain questionnaire.
Results
Both lidocaine (1 mg/kg, 5 mg/kg) and placebo significantly reduced the intense, sharp, hot, dull, cold, sensitivity, itchy, unpleasant, deep and superficial of pain. The amount of change was not significantly different among either of the lidocaine and placebo, or among the lidocaine treatments themselves, for any of the pain responses, except sharp, dull, cold, unpleasant, and deep pain. And VAS was decreased during infusion in all 3 group and there were no difference among groups.
There are many cause of failed back surgery syndrome (FBSS) [1]. The etiology is that about 58% lateral canal stenosis, 7-14% central stenosis, 12-16% recurrent disc hernation, 6-16% arachnoiditis, and 6-8% epidural fibrosis [2]. Other less common causes include nerve injury during surgery (neuropathic pain), chronic mechanical pain, painful segment disc above a fusion, pseudoarthrosis, foreign body, and surgery performed at the wrong level.
Among those causes, neuropathic pain observed 5-9% in patients [2,3]. There are several potential mechanisms for neuropathic pain after spine surgery. A nerve root could have been damaged prior to surgery because of either sudden injury or prolonged compression from foraminal stenosis or disc herniation. Alternatively, a nerve could be damaged during the surgery itself. After then, an ectopic impulse generated within injured nerve or dorsal root ganglion. These abnormal pulses may be associated with neuropathic pain.
In generally, tricyclic antidepressants, dual reuptake inhibitors of serotonin and norepinephrine, calcium channel alpha(2)-delta ligands (i.e, gabapentin and pregabalin), and topical lidocaine were recommended as first-line treatment options on the basis of the results of randomized clinical trials [4].
An intravenous infusion of lidocaine has been used on numerous occasions to produce analgesia in neuropathic pain [5-7]. In the cases of FBSS, the pain generated as result of abnormal impulse from the dorsal root ganglion and spinal cord, for instance as a result of nerve injury may be particularly sensitive to lidocaine [8-10].
Lidocaine is a local anesthetic of the amide type sodium channel blocker and produces analgesia by blockade of peripheral and central sodium ion gate channels, including the spinal dorsal horn [10] or inhibition of neural ectopic discharges [11].
A variable pain qualities and quantities reflect differing underlying pain mechanisms and might therefore predict differing response to treatment [12]. In previous study, the pain intensity reductions in response to intravenous lidocaine are different according to pain qualities [13]. Also a open label clinical trial of lidocaine patch 5% for chronic neuropathic and nociceptive pain provide different reduction of each of the neuropathic pain scale items [14].
However, no previous studies have previously addressed the efficacy of IV lidocaine on the neuropathic items in patients with failed back surgery syndrome (FBSS).
The aim of the present study was to evaluate the efficacy of IV lidocaine on pain and its specific effects on the neuropathic pain scale in patients with neuropathic pain caused by lumbar surgery.
We performed a randomized, prospective, double-blinded, crossover study involving eighteen who received three treatments. The study was conducted with the full approval of the Institutional Review Board. All patients had long-standing (>2 years) neuropathic pain; neuropathic pain suspected by a board certified specialist who assessed the patients based on the presence of allodynia, hyperalgesia, hyperpathia, hyperesthesia and hypoesthesia. Inclusion criteria for FBSS were: 1) the patients had undergone lumbar surgery. 2) MRI showed no evidence of anatomical abnormalities (stenosis, recur, arachnoiditis, and instability); 3) EMG showed neuropathy; 4) all patients had to have failed standard pharmacological or interventional treatments, failure was defined as: 1) no response to treatment or; 2) no lasting relief of pain (<2 months); and 3) persistence, progression of the syndrome.
Patients with known contraindication such as allergies to lidocaine, seizure disorder, a history of substance or drug abuse, psychiatric illness, or suspected somatoform pain disorder were excluded. And a history of gastric or duodenal ulcer, vascular disease, arrhythmia, ischemic heart disease, or renal insufficiency was excluded.
Eighteen patients received each of following intravenous infusion over 1 hour at 2 weeks apart: normal saline placebo, lidociane 1 mg/kg, and lidocaine 5 mg/kg in 500 ml normal saline at 60 ml/hr initially, and then titrated infusion speed while keep the heart rate < 130 rates/min or 160 mmHg > systolic blood pressure > 85 mmHg.
The patient underwent infusions on three different days. Each day, the subject received one of the three infusions in randomized order. Both the patient and person doing the pain evaluations were blinded to the drug infused. Pain intensity was assessed using visual analogue scale (VAS) (0-10/10, 0; no pain, 10: the most pain) and neuropathic pain scales (NPS). A VAS scores were measured at just before injection, at every 10 minutes up to 60 minutes during injection, and then at 8 hrs, 16 hrs, and 24 hrs after injection (The subjects were asked to take home a diary in which they would report on VAS).
During the infusions, the subjects' blood pressure was recorded every 5 minutes and electrocardiography,was monitored continuously. Following the completion of the study, all patients received a one month follow-up.
All variables are presented as means ± SD. Wilcoxon's signed rank test and the Mann-Whitney test were used for comparison of paired and unpaired data, respectively. Repeated measures analysis of variance (ANOVA) was used for comparisons of VAS. A P < 0.05 was considered significant for all analyses. SPSS version 17 was used for all analyses (SPSS Inc., Chicago, IL).
Eighteen patients completed the study. Of these, thirteen (72%) were female. Descriptive statistics are shown in Table 1.
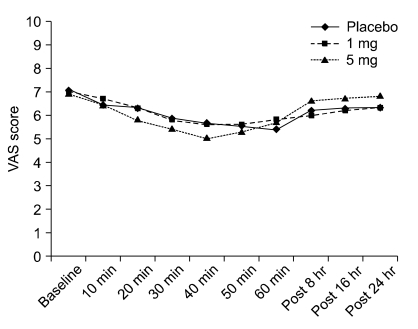
The effect of lidocaine infusion on neuropathic pain is shown in Table 2, and Fig. 1. Figure 1 shows the VAS scores for pain plotted against the time of the three infusions. The VAS scores before starting were compared to the score at the end of the infusion and demonstrated a significant decrease with all three infusions (P = 0.006). Mann-Whitney test demonstrated that there was no difference in VAS scores among the three infusions at either the start of the infusions or the end of the infusions.
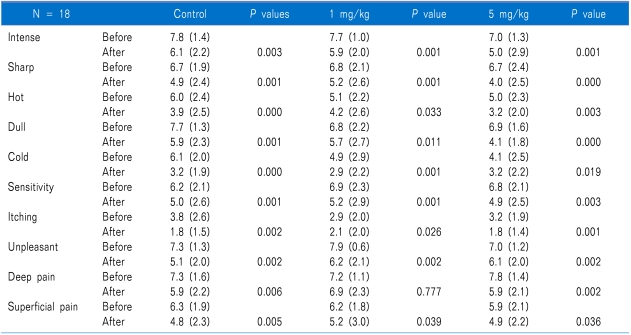
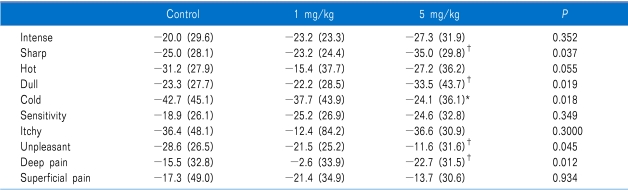
For all 3 treatments, changes from before to after the infusions were significant in NPS (Table 2). The amount of change was not significantly different between either of the lidocaine and placebo, or between the lidocaine treatments themselves, for any of the pain responses, except sharp, dull, cold, and deep pain (Table 3). Cold pain was most changed in placebo.
This double-blind, placebo-controlled study shows that placebo and IV lidocaine can produce significant analgesic effects and special effects on each items of NPS in a group of patients with neuropathic pain attributable to FBSS.
In the present study, both lidocaine and saline placebo reduced in each of the NPS items. Our expectation was that IV lidocaine and higher dosage of lidocaine would correlate with more reduce of items of NPS and higher pain relief. However, in our study, NPS reduction was achieved in all groups (1 mg/kg or 5 mg/kg of lidocaine and placebo). In addition, the amount of changes in pain after treatment did not differ significantly among the 3 treatments, except for sharp, dull, cold and deep pain. We could not explain this finding. However, after 5 mg/kg, there were significantly changes in sharp, dull, and deep pain. The cold pain was most reduction in saline placebo than lidocaine in this study. One controlled clinical trial reported that infusion of lidocaine at a dose of 5 mg/kg/h significantly decreased of pain intensity compared with placebo [15]. Previous studies have reported results regarding the interaction of pain quality and responsiveness to sodium channel blocker [13]. The patients with high levels of heavy pain quality have significantly a greater decrease in VAS pain intensity [13]. However, our study was inconsistent with this study. We postulated that saline placebo affect to NPS. This may suggest that the mechanism of pain was heterogeneous in spite of the single disease.
The analgesic effects of lidocaine observed in our patients did not different among 1 mg/kg and 5 mg/kg of lidocaine.Results of the present study which VAS was decreased correspond with the results of earlier studies [6,7,16,17]. This suggests that lidocaine produces a dose-related reduction of afferent activity from dorsal root ganglion [8-10]. In addition, analgesia produced by lidocaine appears to result from suppression of tonic neural discharge in injured peripheral A-delta and C fiber nociceptors [8].
The effective dose range of systemic lidocaine is comparable among different neuropathic pain conditions. The range of the lidocaine plasma level is from 0.62 to 5.0 µg/ml [18]. It may be that a minimum therapeutic plasma level for lidocaine was reached at 1 mg/kg of lidocaine [7]. Moreover, the free concentration of lidocaine showed no better correlation with the onset of analgesia or the attainment of complete analgesia than the serum concentration of lidocaine. Ferrante et al. [17] suggested that the mechanism of analgesia from IV lidocaine is not being based upon a conventional concentration-effect relationship and there was not a direct relationship between total or free lidocaine levels and the effect on neuropathic pain [15]. However, we did not determine the blood levels of lidocaine.
In the present study, equal pain reduction was seen in both lidocaine and the saline placebo. It is possible that because of the small sample studied, we cannot entirely exclude the possibility that lack of difference between lidocanie and placebo is due to a type 2 error. In Baranowski et al's study [7] there was a significant reduction in constant pain with placebo and he suggested that constant pain occurs less as a result of peripheral stimulation. Furthermore Tremont-Lukats et al. [15] reported that lidocaine at lower infusion rates (1 mg/kg, and 3 mg/kg) was no better than placebo in relieving pain. However, the author did not conclude that doses of lidocaine lower than 5 mg/kg/h are not effective and not different from placebo. In painful neuropathy in cancer patients study [16], neither lidocaine (5 mg/kg) nor placebo reduce pain intensity or consumption of analgesics significantly. The author suggested that it is a different effect on mechanical and other somatosensory input by touch stimuli still evoking activity in the dorsal horn cell.
There were several limitations to the present study. Fisrt, we identified were not corroborated with any complications, Second, follow-up was less than 24 hours, so there were no result from mid- or long-term follow periods.
In conclusion, this study shows that 1 mg/kg, or 5 mg/kg of IV lidocaine, and placebo was effective to patients with neuropathic pain attributable to FBSS, but effect of lidocaine did not differ from placebo. We did not explain placebo effect. Further adequately powered trials should be established whether saline have same with lidocaine.
References
2. Waguespack A, Schofferman J, Slosar P, Reynolds J. Etiology of long-term failures of lumbar spine surgery. Pain Med. 2002; 3:18–22. PMID: 15102214.

3. Burton CV, Kirkaldy-Willis WH, Yong-Hing K, Heithoff KB. Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res. 1981; 157:191–199. PMID: 7249453.

4. Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010; 85:S3–S14. PMID: 20194146.

5. Tremont-Lukats IW, Challapalli V, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth Analg. 2005; 101:1738–1749. PMID: 16301253.

6. Attal N, Gaudé V, Brasseur L, Dupuy M, Guirimand F, Parker F, et al. Intravenous lidocaine in central pain: a double-blind, placebo-controlled, psychophysical study. Neurology. 2000; 54:564–574. PMID: 10680784.

7. Baranowski AP, De Courcey J, Bonello E. A trial of intravenous lidocaine on the pain and allodynia of postherpetic neuralgia. J Pain Symptom Manage. 1999; 17:429–433. PMID: 10388248.

8. Tanelian DL, MacIver MB. Analgesic concentrations of lidocaine suppress tonic A-delta and C fiber discharges produced by acute injury. Anesthesiology. 1991; 74:934–936. PMID: 2021210.

9. Chabal C, Russell LC, Burchiel KJ. The effect of intravenous lidocaine, tocainide, and mexiletine on spontaneously active fibers originating in rat sciatic neuromas. Pain. 1989; 38:333–338. PMID: 2510116.

10. Woolf CJ, Wiesenfeld-Hallin Z. The systemic administration of local anaesthetics produces a selective depression of C-afferent fibre evoked activity in the spinal cord. Pain. 1985; 23:361–374. PMID: 3937116.

11. Nagy I, Woolf CJ. Lignocaine selectively reduces C fibre-evoked neuronal activity in rat spinal cord in vitro by decreasing N-methyl-D-aspartate and neurokinin receptor-mediated post-synaptic depolarizations; implications for the development of novel centrally acting analgesics. Pain. 1996; 64:59–70. PMID: 8867247.

12. Jensen MP. Using pain quality assessment measures for selecting analgesic agents. Clin J Pain. 2006; 22:S9–S13. PMID: 16344610.

13. Carroll IR, Younger JW, Mackey SC. Pain quality predicts lidocaine analgesia among patients with suspected neuropathic pain. Pain Med. 2010; 11:617–621. PMID: 20210867.

14. Jensen MP, Dworkin RH, Gammaitoni AR, Olaleye DO, Oleka N, Galer BS. Assessment of pain quality in chronic neuropathic and nociceptive pain clinical trials with the Neuropathic Pain Scale. J Pain. 2005; 6:98–106. PMID: 15694876.

15. Tremont-Lukats IW, Hutson PR, Backonja MM. A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. 2006; 22:266–271. PMID: 16514327.

16. Ellemann K, Sjögren P, Banning AM, Jensen TS, Smith T, Geertsen P. Trial of intravenous lidocaine on painful neuropathy in cancer patients. Clin J Pain. 1989; 5:291–294. PMID: 2520418.

17. Ferrante FM, Paggioli J, Cherukuri S, Arthur GR. The analgesic response to intravenous lidocaine in the treatment of neuropathic pain. Anesth Analg. 1996; 82:91–97. PMID: 8712433.

18. Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000; 87:7–17. PMID: 10863041.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download