Abstract
Intrathecal drug administration system (ITDAS) can reduce the side effects while increasing the effectiveness of opioids compared to systemic opioid administration. Therefore, the use of ITDAS has increased in the management of cancer pain and chronic intractable pain. Catheter obstruction is a serious complication of ITDAS. Here, we present a case of catheter obstruction by a mass formed at the side hole and in the lumen. A 37-year-old man suffering from failed back surgery syndrome received an ITDAS implantation, and the ITDAS was refilled with morphine every 3 months. When the patient visited the hospital 18 months after ITDAS implantation for a refill, the amount of delivered morphine sulfate was much less than expected. Movement of the pump rotor was examined with fluoroscopy; however, it was normal. CSF aspiration through the catheter access port was impossible. When the intrathecal catheter was removed, we observed that the side hole and lumen of the catheter was plugged.
Go to : 
Intrathecal injection of opioids has the same pain control effect with 1/300 quantity of oral administration; therefore, it can reduce complications arising from the use of opioids [1]. The intrathecal drug administration system (ITDAS) is an instrument that can continually inject a programmed amount of opioids for a long period of time and was first used for pain management in cancer patients in 1981 [2]. In 1982, the Infusaid pump® was the first commercialized implantable infusion pump (Shiley Infusaid Inc., Norwood, MA, USA) [3], and in 1991, the SynchroMed® pump (Medtronic Inc., Minneapolis, MN, USA), which is programmable, received FDA approval in the United States [4].
Recently, the procedure has been performed in patients with non-cancer pain that cannot be controlled by other treatment such as failed back surgery syndrome, complex regional pain syndrome, postherpetic neuralgia, and peripheral neuropathy [5]. This non-cancer pain lasts for longer periods compared to the pain from cancerous diseases; thus, the follow-up observation period after inserting the ITDAS becomes longer with a higher risk of more complications.
Regarding the complications of ITDAS, catheter obstruction is a very serious complication, and there are clinical recommendations on the concentration and quantity of opioids to prevent its occurrence [6]. Despite following the recommendations that prevent catheter tip mass formation, the authors experienced a case where the catheter was obstructed and drug administration was stopped because of a mass in the side hole and lumen and hence, are reporting this case with a literature review.
A 37-year-old male patient 163 cm tall weighing 45.6 kg visited the hospital with paraplegia, voiding difficulty, hypesthesia below L1, and pain with a visual analogue scale (VAS) score 8/10 as post lumbar surgery syndrome, which occurred after receiving surgery for congenital scoliosis. Twenty-four months prior to his visit, he had received treatment from an another hospital, which included caudal epidural block and epidural adhesiolysis, and medication including transdermal fentanyl patch (Durogesic D-trans®, Janssen Korea Ltd., Seoul, Korea) 37 mcg/h, oxycodone hydrochloride (Oxycontin CR®, Mundipharma Korea Ltd., Seoul, Korea) 40 mg/d, Ultracet® (Janssen Korea Ltd., Seoul, Korea) 4 tablets/d, and pregabalin (Lyrica®, Pfizer Korea Inc., Seoul, Korea) 600 mg/d was prescribed; however, there was no improvement in his pain. Hence, 20 months before visiting our hospital, the patient had an ITDAS (SynchroMed® II, Medtronic Inc., Minneapolis, MN, USA) inserted with the catheter tip positioned at the L1 body height. After the procedure, the ITDAS was refilled with 18 ml of morphine sulfate (Highmol® 10 mg/ml, BCWorld Pharm. Co., Korea) every three months, and the injected quantity was maintained between 0.95-1.7 mg/d, and the pain was controlled with a VAS score of 3-4/10.
Fifteen months after inserting the ITDAS, the remaining medication was removed to refill the device; however, 17 ml of morphine sulfate was left in the device, which should have been 5.6 ml had the injections been normal. Other than increased pain with a VAS score of 8/10, there were no neurological abnormalities that had newly occurred, and the test program within the instrument was functioning normally without any failures. The patient had undergone surgery in the left elbow joint two months prior; thus, a temporary software problem due to the monopolar electrocautery during the surgery was believed to be the cause of the problem. After disposing the remaining medication, another 18 ml of morphine sulfate was refilled, and the program was reset. At that time, the patient's pain had not controlled; therefore, oxycodone hydrochloride (IRcodon®, Mundipharma Korea Ltd., Seoul, Korea) 30 mg/d was taken by the patient whenever there was pain. A transdermal fentanyl patch (Durogesic D-trans®, Janssen Korea Ltd., Seoul, Korea) 25 mcg/h was additionally prescribed.
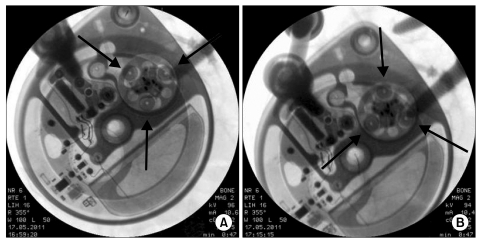
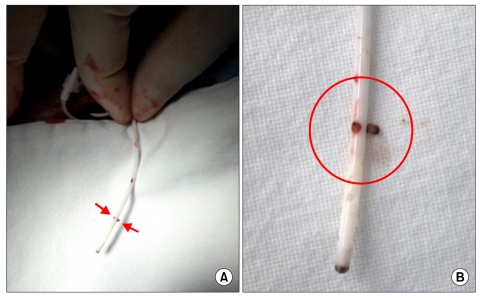
Three months later, when the patient visited the hospital to refill the device, the 18 ml of morphine sulfate were still in the device, and the test program in the instrument was functioning normally. To examine whether the instrument was operating properly, priming bolus mode was initiated, and 15 minutes later, the movement of the rotor was confirmed under the fluoroscope, which confirmed it was operating normally (Fig. 1). Therefore, it seemed that there could be a catheter obstruction rather than problems with the instrument itself; therefore, it was decided to aspirate some of the cerebrospinal fluid from the catheter's access port. The total volume of the catheter was calculated as 0.132 ml; thus, a 1 ml needle was used. The needle was inserted into the access port and aspiration was attempted, but the authors were unable to aspirate any of the cerebrospinal fluid. An emergency kit was prepared and 0.05 ml of normal saline was injected into the catheter's access port; however, it was unable to enter the port. Another attempt was made with 0.05 ml, but there was strong pressure and the normal saline did not go in at all. Catheter kinking or blockage by a mass was suspected; therefore, the decision was made to extract the catheter for confirmation. The catheter and instrument were extracted, and it was confirmed that the catheter's side hole was blocked by a tissue mass, and it was also observed that the catheter's lumen was blocked (Fig. 2). We had planned to exchange the catheter, but the patient and his wife were worried about repeated occurrences of the catheter being blocked. They wanted to remove the ITDAS; therefore, the ITDAS was completely removed. At present, the pain is maintained at a VAS score of 6/10 with a transdermal fentanyl patch (Durogesic D-trans®, Janssen Korea Ltd., Seoul, Korea) 50 mcg/h, pregabalin (Lyrica®, Pfizer Korea Inc., Seoul, Korea) 600 mg/d, tramadol hydrochloride 100 mg/d (Tramaconti®, Whanin Pharm co., Seoul, Korea), and oxycodone hydrochloride (Oxycontin CR®, Mundipharma Korea Ltd., Seoul, Korea) 40 mg/d.
Go to : 
An intrathecal mass that occurred in the tip of an ITDAS catheter was first reported in a patient with chronic intractable pain in 1991 [7]. The formation of granulomas in intrathecal catheters has been reported to have a 0.04% occurrence rate two years after insertion of the catheter and 1.15% six years after insertion [8]. Granulomas are a result of responses to local inflammation due to the activation of endothelial cells, granulocytes, and monocytes [9]. Granulomas are caused by active drugs, preservatives, indolent organisms, changes in pH, the material of the catheter (silicon), and injury during insertion [10]. Masses that form at the catheter tip press on the spinal cord to cause neurological abnormalities, and when treatment is delayed, it may cause permanent damage to the spinal cord [8].
Symptoms from granulomas are motor weakness, sensory loss, changes in reflex functions, and bladder dysfunction. Other symptoms can be numbness, tingling, burning, hyperesthesia, hyperalgesia, and radicular pain at the same level as the catheter tip. A granuloma is suspected when the required amount of opioids increases for the same amount of analgesia effect and pain control [11]. When neurological abnormalities progress rapidly, a granuloma should be surgically removed as soon as possible for a better prognosis, and even though neurological abnormalities may have only occurred for a short period, delays in treatment can make recovery difficult; thus, surgery should be performed promptly [7,12]. When the neurological disorder is not severe or when the pressure from the mass on the nerve is weak, it can be treated without surgical removal. If administration of the drug is stopped or the medication is replaced with normal saline, the size of granuloma can become smaller within 2-5 months [12]. Neurological examination should be performed before inserting the ITDAS, and regular examinations should be performed after insertion to detect neurological abnormalities early on so it can be treated with relatively simple methods such as stopping the administration of the medication rather than invasive methods such as surgery. In our case, no new neurological abnormalities occurred besides increased pain. In addition, since an MRI could not be performed due to the economic situation of the patient in our case, and the test program within the instrument indicated normal functioning, we suspected catheter obstruction based on increased pain, reduced administration of morphine sulfate lower than the programmed amount, and the observation of normal movement of the rotor with fluoroscopy. Inserting a needle into the CAP for catheter patency check and performing dye injection after CSF aspiration could be considered, but according to the experience of the authors, the inserted catheter's length was 60-80 cm long; thus, it would be very difficult to suction CSF through the CAP. Additionally, as in our case, the movement of the rotor must be checked because due to the structure of the SynchroMed II®; the force that administers the medication is from pressurized gas, which pressurizes the bellows type drug reservoir to push the medication into the catheter, and the rotor only has the role of administering the exact amount of medication. In other words, the rotor is unrelated to the propulsion and simply acts as to precisely control the quantity of medication administered.
Various methods to prevent the formation of masses in the ITDAS catheter tip have been introduced. First, since the concentration and dose of the medication is higher and the flow rate is lower, there is a high possibility of mass formation; thus, it is recommended that the maximum concentration should be 20 mg/ml, and the maximum dose should not to exceed 15 mg/d [6]. Since the CSF space is wider, it lowers the possibility of a granuloma; therefore, it is best to position the catheter tip at T7-T10 or L1-L2 where the CSF space is wide [6]. If the catheter tip is located lower than the conus medullaris, it can minimize neurological abnormalities even when a mass is formed [11]. However, in lipophilic drugs such as baclofen, the catheter tip should be located near the targeted spinal nerve, but morphine is a hydrophilic drug so placing it lower than the conus medullaris can reduce complications. In our case, a mass still formed despite an inserted morphine concentration of 10 mg/ml, used dose 107 mg/d, and the catheter tip being located at L1. Therefore additional methods such as regular flushing should be considered to prevent mass formation. Moreover, the catheter used in our case was a side-hole type; therefore, if end-hole types are used in the future, this may help to reduce mass formation.
In conclusion, to prevent complications from mass formation in a catheter tip, a suitable location for the catheter should be selected at the time of ITDAS insertion, and the administered dose and concentration of morphine should be as low as possible. In addition, to detect a catheter obstruction early on, under-infusion must be checked at the time of medication refill. When rotor movement is normal, the possibility of a catheter obstruction must be considered.
Go to : 
References
2. Onofrio BM, Yaksh TL, Arnold PG. Continuous low-dose intrathecal morphine administration in the treatment of chronic pain of malignant origin. Mayo Clin Proc. 1981; 56:516–520. PMID: 6894954.

3. Johnston J, Reich S, Bailey A, Sluetz J. Shiley INFUSAID Pump technology. Ann N Y Acad Sci. 1988; 531:57–65. PMID: 3382145.

4. Belverud S, Mogilner A, Schulder M. Intrathecal pumps. Neurotherapeutics. 2008; 5:114–122. PMID: 18164490.

5. Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996; 11:71–80. PMID: 8907137.

6. Deer T, Krames ES, Hassenbusch S, Burton A, Caraway D, Dupen S, et al. Management of intrathecal catheter-tip inflammatory masses: an updated 2007 consensus statement from an expert panel. Neuromodulation. 2008; 11:77–91. PMID: 22151039.

7. North RB, Cutchis PN, Epstein JA, Long DM. Spinal cord compression complicating subarachnoid infusion of morphine: case report and laboratory experience. Neurosurgery. 1991; 29:778–784. PMID: 1961414.

8. Yaksh TL, Hassenbusch S, Burchiel K, Hildebrand KR, Page LM, Coffey RJ. Inflammatory masses associated with intrathecal drug infusion: a review of preclinical evidence and human data. Pain Med. 2002; 3:300–312. PMID: 15099235.

9. Magazine HI, Liu Y, Bilfinger TV, Fricchione GL, Stefano GB. Morphine-induced conformational changes in human monocytes, granulocytes, and endothelial cells and in invertebrate immunocytes and microglia are mediated by nitric oxide. J Immunol. 1996; 156:4845–4850. PMID: 8648133.
10. Miele VJ, Price KO, Bloomfield S, Hogg J, Bailes JE. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006; 10:251–261. PMID: 15964775.

11. Hassenbusch S, Burchiel K, Coffey RJ, Cousins MJ, Deer T, Hahn MB, et al. Management of intrathecal catheter-tip inflammatory masses: a consensus statement. Pain Med. 2002; 3:313–323. PMID: 15099236.

12. Coffey RJ, Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: report and observations on 41 patients. Neurosurgery. 2002; 50:78–86. PMID: 11844237.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download