Abstract
The Nuss procedure for the correction of Pectus Excavatum (PE) is associated with intense postoperative pain. Our strategy to control early postoperative pain is to combine epidural with intravenous analgesia. Our aim was to analyse our pain control strategy by reviewing all the PE cases treated at our institution. Sixty consecutive patients, aged between 12 and 26 years old, received the PE operation at our institution from January, 2007 to September, 2010. The median age was 16 (12-27) with a male/female ratio of about 7/1. An epidural catheter was employed in all the cases, with 38 patients (63%) requiring additional drugs to control pain, which remained in place for 74 hours (72-96). The pain score was higher in male patients, but lower in those younger than 16 years old. Moreover, patients that consumed benzodiazepines had a significant decrease in cumulative opioid intake (P = 0.0408). Both gender and age had an impact on pain control, while we noticed a synergistic effect between opiates and tranquillizers.
Go to : 
Pectus Excavatum (PE) is the most common form of chest wall deformity found in children, with incidences appearing in approximately 1 out of every 400 births. Although surgical repair of this condition has moved from an overt open procedure towards an assisted thoracoscopic one, popularized as being a minimally invasive repair of PE (MIRPE), it is still burdened with severe postoperative pain [1,2]. The degree of postoperative pain following MIRPE has been shown to be one of the most prevailing factors in assessing the quality of the postoperative course according to patient perceptions. In addition, pain management following MIRPE has been shown to affect all measurable objective outcomes during hospitalization, which includes the capacity for deep breathing, the capacity of early mobilization, the possibility to perambulate, and the length of hospital stay [3]. Epidural analgesia (EA) has been reported as being one of the standard methods for managing pain in the early postoperative period after PE repair [4-6]. However, in these patients, severe pain might persist even after the removal of the epidural catheter [7,8]. To date, there is still an open debate on which type of analgesia should be undertaken for this procedure; there is no consistent data available about pain treatment after the discontinuation of EA, nor for how long EA should take place [7,9,10]. The purpose of this study was to describe the entire management process for postoperative pain in 60 patients who were submitted to undergo MIRPE, starting from the early postoperative period during the EA regimen, through the critical transitional period between epidural and oral analgesia and the full oral pain medication plan. The secondary aim of this study was to determine the influence of benzodiazepines on the opioid consumption after the Nuss procedure.
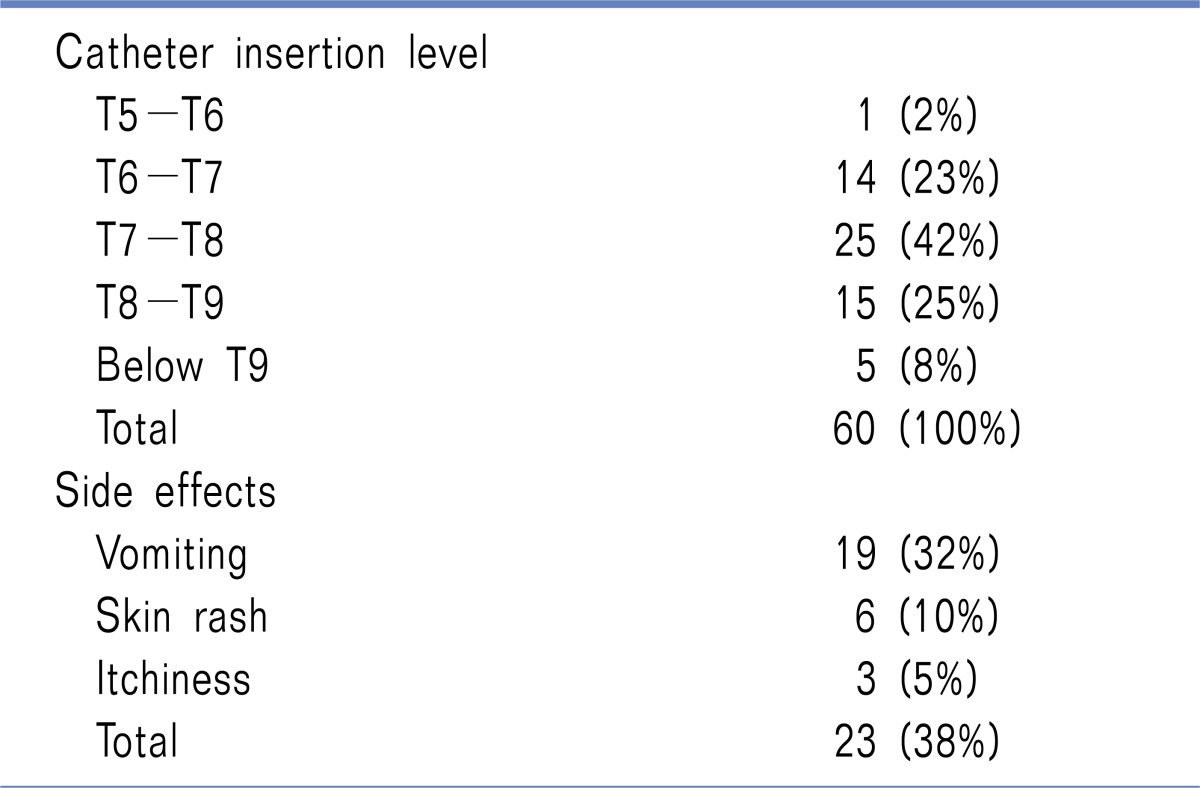
Sixty patients between 12 and 26 years of age underwent MIRPE. The male/female ratio was approximately 7 : 1. The mean weight was 59 kg (35-83). The Haller index ranged from 2.6 to 13, with a median value of 5.0. Thirty two patients (53%) had an asymmetric profile. All patients received oral benzodiazepines (lorazepam 0.02-0.1 mg/kg/dose) the night before the surgery. The mean duration of anaesthesia was 2.9 hours (2.3-3.2) and the time for surgery ranged between 1 to 3 hours, with a median duration of 1.1 hours. EA was activated with 0.375% levobupivacaine and a clonidine 1-2 ug/kg injection, and as per protocol, continuous epidural infusion of fentanyl 0.2-0.4 ug/kg/h in 0.1% levobupivacaine associated with intravenous paracetamol 15-20 mg/kg/dose was administered at the end of the operation. During EA, 23 patients (38%) experienced side effects as reported in the table (Table 1). The epidural catheter was kept in place for 74 hours (72-96). Malfunctioning, which required catheter removal, was presented in 5 patients (8%) due to obstruction. Additional drugs (ketorolac, morphine, tramadol) were administered when necessary (NRS > 4).
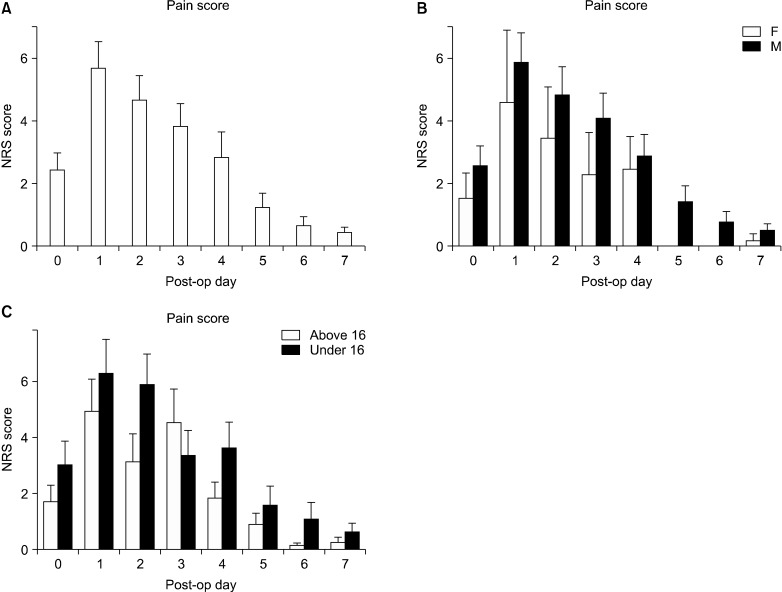
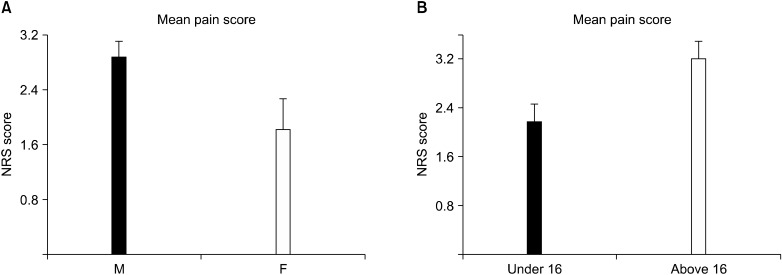
Thirty-eight patients (63%) required additional analgesics during the EA period. After EA suspension, patients received both slow release oral oxycodone (starting with 10 mg twice/day then reduced to 5 mg once/day) and oral paracetamol (15-20 mg/kg/dose). The additional i.v. drugs that were the same (ketorolac, morphine, tramadol) were administered when required (NRS > 4). During this period, 43 patients (72%) required additional analgesics, mostly during the critical change over phase from the EA to the oral analgesia regimen. The average duration of oral medication was 5.5 ± 3 days. Patients were discharged with oral therapy if required (paracetamol 500 mg and codeine phosphate 30 mg). The length of hospital stay was 10 days (8-29). The daily pain score assessments, via NRS, demonstrated that pain increased since the operative day with a maximum being reached in the first postoperative day (Fig. 1A). The following postoperative days, the pain score decreased progressively, reaching an NRS below 4 after the fourth postoperative day (Fig. 1). Both gender and age seemed to have a significant overall impact on the mean pain experienced: P = 0.0417 and P = 0.0199, respectively (Fig. 2). Pectus severity did not correlate with the pain level though (P > 0.05 - data not shown).
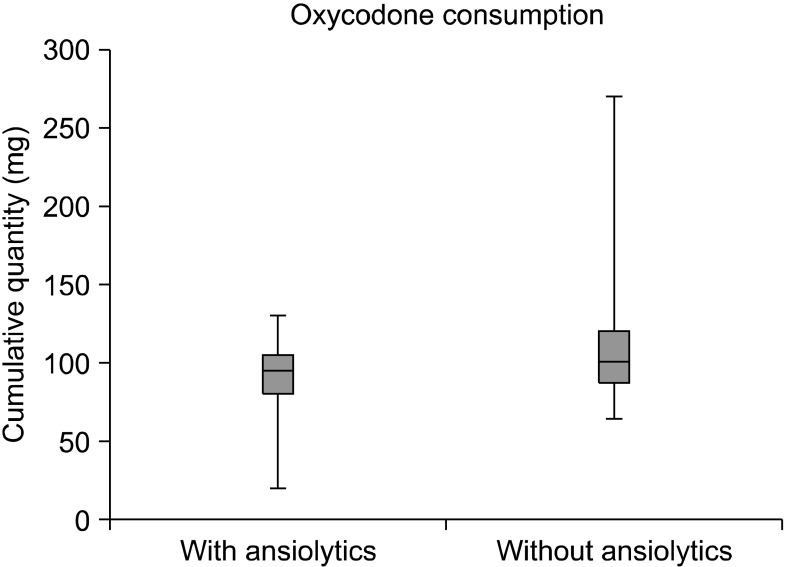
During hospitalization, additional oral benzodiazepines were administered in 19 patients when highlighted with an anxiety condition (32%). Cumulative oral opiates dose was 97.5 mg (20-270). Patients who consumed benzodiazepines had a significant decrease in postoperative oral oxycodone intake in comparison to those who had just oral analgesia, which were administered respectively at 90 mg and 109 mg in dosage (P = 0.0408) (Fig. 3). Comparative groups were established based on, age (patients > 16 years-old and patients ≤ 16 years-old), gender (male and female), and eventually on anxiety management (administration of benzodiazepines and no benzodiazepines in association with oxycodone). Groups were compared by means of the Student t-test for unpaired values and ANOVA. A P value < 0.05 was considered statistically significant.
Go to : 
In this study, we provided the description of the entire management process of postoperative pain at our institution for a consecutive group of patients submitted for MIRPE. We evaluated the patients from the moment the epidural catheter was inserted, while following the critical transitional period between epidural and oral analgesia, concluding with the complete oral pain medication regimen until discharge. Moreover, we analyzed the influence of benzodiazepines on the cumulative opioid intake after surgery.
Pain is undoubtedly a dominant factor that determines the quality of the postoperative course of patients with pectus bar placement. By abruptly elevating the sternum anteriorly, a substantial amount of pain in the chest and back of the skeletally mature patient is generated. The increased pressure on the bar, due to a less plastic chest, increases the magnitude of pain which involves dermatomes T1-10 [10]. In our study, a group of patients who underwent MIRPE was, to some extent, older in comparison to several other reports . This discrepancy reflects the rationale of subjecting patients to PE correction only if the patients themselves demand the surgical correction [1,6,11]. An adequate peri- and postoperative pain management regiment has become an important part of thoracic anaesthesia. EA has proven to be effective for thoracic procedures, which have an intense pain as a consequence of the surgery, and is currently proposed for MIRPE [7,12].
The opioids currently in use for EA, such as Fentanyl, being lipophilic with a proportional short half-life, have limited rostral spread and less respiratory depression than morphine [13]. Opioids have been associated with local anaesthetics to block spinal nociceptive pathways in the epidural administration, thus reducing the dose-related adverse effects of both classes of drugs [12,13]. In our series, both the duration of EA and oral medication administration were, to some extent, in line with previous studies [11,14]. However, in contrast to some reports, during both the EA period and after the removal of the epidural catheter, most of our patients required additional analgesics, despite the regular administration of a strong oral opioid [4,9]. Regarding the adverse effects during EA therapy and the incidences of accidental premature discontinuation of the epidural catheters, due mainly to block failure, our data are in accordance with previous studies [2]. Concerning pain control, we observed a slow but progressive improvement in the pain score over time, with a reduction in score below 4 being considered as the threshold for rescue medication, which was determined between the 4th and 5th postoperative day. Both gender and age seemed to have an overall significant impact on the pain score, which decreased in the female population and increased in older patients. The latter most likely reflects the difference in anatomical plasticity and moldability of the rib cage in early adolescents to that found at adult age [10]. Interestingly enough, pectus severity did not correlate with the pain level experienced by our patients.
Benzodiazepines are commonly used for a wide range of conditions and are known to have sedative, hypnotic, anticonvulsant, muscle relaxant, and amnesic properties, making them useful in treating anxiety. Clinical research supports a tight relationship between psychological factors like anxiety and pain. Anxiety may increase sensitivity and the intensity of pain, which in turn influences the anxiety state into a self-maintaining mechanism. Anxiety that follows pain determines a stressful condition, with various symptoms, such as muscle tension, which cause a reduction of the pain threshold. In this context, anxiolytic drugs may have a beneficial role by giving the patient a reduced sensation of pain in the postoperative period, thanks to the mutual reinforcement mechanism between anxiety and pain [15]. From our limited experience, we confirm that postoperative oxycodone consumption, during the oral drug intake period, was reduced in the group of patients that used benzodiazepines conjunctively.
In summary, with this retrospective study, we provided additional data regarding pain management after the surgical repair of PE. Our results support the efficacy of EA. The current data showed good overall pain control, the need of five or more days of oral medication with strong opioids after the epidural discharge, and additional analgesic administration if requested by the patients both during the intravenous and oral analgesic period. We also assumed that, for better pain management, it is advisable to evaluate the pain and anxiety state during hospitalization, which was thought to be necessary when taking into consideration postoperative anxiolytic treatment of patients after the Nuss procedure. In any case, given these preliminary results, a prospective study is warranted in order to establish the value of the routine use of benzodiazepines in MIRPE, especially during the first postoperative days when postoperative pain is most severe.
Go to : 
References
1. Grosen K, Pfeiffer-Jensen M, Pilegaard HK. Postoperative consumption of opioid analgesics following correction of pectus excavatum is influenced by pectus severity: a single-centre study of 236 patients undergoing minimally invasive correction of pectus excavatum. Eur J Cardiothorac Surg. 2010; 37:833–839. PMID: 19853467.

2. Rugyte DC, Kilda A, Karbonskiene A, Barauskas V. Systemic postoperative pain management following minimally invasive pectus excavatum repair in children and adolescents: a retrospective comparison of intravenous patient-controlled analgesia and continuous infusion with morphine. Pediatr Surg Int. 2010; 26:665–669. PMID: 20490811.

3. St Peter SD, Weesner KA, Sharp RJ, Sharp SW, Ostlie DJ, Holcomb GW 3rd. Is epidural anesthesia truly the best pain management strategy after minimally invasive pectus excavatum repair? J Pediatr Surg. 2008; 43:79–82. PMID: 18206460.

4. Futagawa K, Suwa I, Okuda T, Kamamoto H, Sugiura J, Kajikawa R, et al. Anesthetic management for the minimally invasive Nuss procedure in 21 patients with pectus excavatum. J Anesth. 2006; 20:48–50. PMID: 16421678.

5. Weber T, Mätzl J, Rokitansky A, Klimscha W, Neumann K, Deusch E, et al. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patientcontrolled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg. 2007; 134:865–870. PMID: 17903498.

6. Soliman IE, Apuya JS, Fertal KM, Simpson PM, Tobias JD. Intravenous versus epidural analgesia after surgical repair of pectus excavatum. Am J Ther. 2009; 16:398–403. PMID: 19262363.

7. Cucchiaro G, Adzick SN, Rose JB, Maxwell L, Watcha M. A comparison of epidural bupivacaine-fentanyl and bupivacaine-clonidine in children undergoing the Nuss procedure. Anesth Analg. 2006; 103:322–327. PMID: 16861412.

8. Walaszczyk M, Knapik P, Misiolek H, Korlacki W. Epidural and opioid analgesia following the Nuss procedure. Med Sci Monit. 2011; 17:PH81–PH86. PMID: 22037752.

9. Densmore JC, Peterson DB, Stahovic LL, Czarnecki ML, Hainsworth KR, Davies HW, et al. Initial surgical and pain management outcomes after Nuss procedure. J Pediatr Surg. 2010; 45:1767–1771. PMID: 20850618.

10. Yapici D, Atici S, Alic M, Ayan E, Koksel O. Morphine added to local anaesthetic improves epidural analgesia in minimally invasive Nuss operation for pectus excavatum. Br J Anaesth. 2008; 100:280. PMID: 18212003.

11. Al-Assiri A, Kravarusic D, Wong V, Dicken B, Milbrandt K, Sigalet DL. Operative innovation to the "Nuss" procedure for pectus excavatum: operative and functional effects. J Pediatr Surg. 2009; 44:888–892. PMID: 19433163.

12. Reinoso-Barbero F, Fernández A, Durán P, Castro LE, Campo G, Melo MM. Thoracic epidural analgesia vs patientcontrolled analgesia with intravenous fentanyl in children treated for pectus excavatum with the Nuss procedure. Rev Esp Anestesiol Reanim. 2010; 57:214–219. PMID: 20499799.

13. Butkovic D, Kralik S, Matolic M, Kralik M, Toljan S, Radesic L. Postoperative analgesia with intravenous fentanyl PCA vs epidural block after thoracoscopic pectus excavatum repair in children. Br J Anaesth. 2007; 98:677–681. PMID: 17363405.

14. Cartoski MJ, Nuss D, Goretsky MJ, Proud VK, Croitoru DP, Gustin T, et al. Classification of the dysmorphology of pectus excavatum. J Pediatr Surg. 2006; 41:1573–1581. PMID: 16952594.

15. Ozalp G, Sarioglu R, Tuncel G, Aslan K, Kadiogullari N. Preoperative emotional states in patients with breast cancer and postoperative pain. Acta Anaesthesiol Scand. 2003; 47:26–29. PMID: 12492793.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download