Abstract
Several complications are possible after a lumbar epidural block. However pneumocephalus are rare. In this case, we report a case of pneumocephalus. A 68-year-old male patient received lumbar epidural block with the loss of resistance technique using air, and after 35 minutes, apnea, unconsciousness, hypotension, and bradycardia occurred. Immediately, brain CT was done, and we found pneumocephalus. The patient complained of severe occipital headache and itchiness due to pneumocehalus. After conservative treatment, the patient recovered without neurologic complications, and on the seventh day of his hospitalization, he was discharged from the hospital.
Go to : 
Lumbar epidural block is used frequently in the treatment of low back pain. However, after lumbar epidural block, various complications such as hypotension, dizziness, post-dural puncture headache, and transient paraplegia may occur.
Rarely after epidural block, pneumocephalus also might occur. Pneumocephalus is defined as air in the cranial cavity. Although pneumocephalus may occur for many reasons, one that occurs after epidural block is usually caused by the dural puncture performed during the epidural block while applying the loss of resistance technique using air. Thus, when dural puncture is done during epidural block, we can see cerebrospinal fluid (CSF) immediately. Thus, when the patient complains of a headache, we should consider the possibility of post-dural puncture headache or pneumocephalus. However, unless there is CSF leakage, we may have difficulty determining the cause of the headache.
In this case, after we performed the lumbar epidural block with the loss of resistance technique using air, the patient developed symptoms of apnea and unconsciousness. We immediately conducted a brain CT scan on the patient and diagnosed pneumocephalus. Here we report it with a literature review.
A 68-year-old male patient with the height of 172 cm and the weight of 74 kg had been hospitalized in our hospital's dermatology ward for a month due to herpes zoster on his right scalp. He did not improve, so he was transferred to the pain clinic. He received lumbar 4/5 laminectomy and discectomy three months ago, and he had no systemic diseases like hypertension or diabetes. Since then he complained of postherpetic neuralgic symptoms such as spontaneous pain, allodynia, and hyperalgesia. On the fourth day of his hospitalization, he complained of right lower back pain around the region of spinal surgery but with no radicular pain to the lower limb, so we performed a lumbar epidural block.
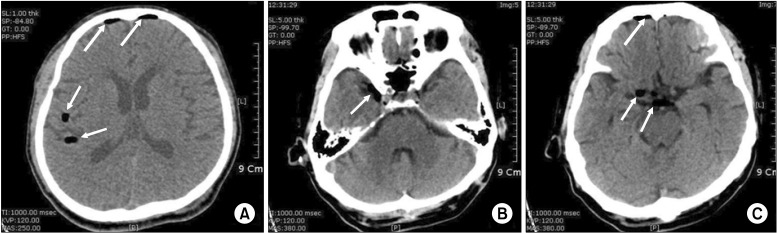
After he took a right decubitus position, axenic disinfection and local infiltration with 2% lidocaine on the region for the procedure were done. We conducted a lumbar epidural block at the lumbar 4/5 interspace with a 20 G Tuohy needle via the median approach. After the loss of resistance using air we confirmed that CSF had not been aspirated before the local anesthetic injection. We injected 0.125% bupivacaine 10 ml with triamcinolone 40 mg. The patient took a rest on the bed for about 30 minutes. He did not show specific symptoms immediately like dizziness, nausea, or vomiting. The patient moved to his room on foot. While walking for about five minutes, he lost consciousness suddenly in front of the elevator near his room. Immediately, we ventilated him manually via facial mask with 100% O2. Vital signs at that moment were: systolic blood pressure (BP) 50 mmHg.; heart rate (HR) 35 beats/min.; there was no voluntary breathing.; saturation was maintained at 98-99% with mask ventilation, and both pupillary reflex were isocoric and prompt. Immediately, we injected 0.5 mg atropine and 20 mg ephedrine intravenously. Five minutes after injection, his vital signs were measured as BP 110/60 mmHg, HR 85 bpm, and SpO2 99%. Fifteen minutes after loss of consciousness, he started to recover consciousness gradually, and after 25 minutes, he recovered perfectly. We found no specific problems in the neurologic examination. The witness said that the patient injured the right temporal area of his head when he lost consciousness and fainted. So even though we found no specific problem outside, we did a CT scan after the patient was stabilized since we could not exclude the possibility of intracranial hemorrhage. The brain CT scan showed pneumocephalus, so we could not rule out skull fracture and did a facial bone CT scan. However, there was no skull fracture (Fig. 1). On arriving at his room after the facial bone CT, he complained of severe occipital headache. 100% oxygen administration via a mask was given to the patient. We injected 75 mg diclofenac sodium intramuscularly. But there was no improvement, so we injected 30 mg ketorolac intravenously. The intravenous ketorolac was also ineffective for the pain. Furthermore, he seemed restless with severe itchiness on his face. We gave him an IV 4 mg of chloropheniramine, but it was not effective at all. So we gave an IV 5 mg of midazolam, which was somewhat effective though not perfect. Five hours after the injection of midazolam, the headache and itchiness disappeared. Only the symptoms resulted from postherpetic neuralgia remained. The next day, the patient showed no specific problems and his vital signs were normal, so he was discharged from the hospital on the seventh day of hospitalization.
Go to : 
After lumbar epidural block, various complications such as hypotension, dizziness, post-dural puncture headache, and motor weakness may occur. Occasionally after epidural block, pneumocephalus also might occur. As in this case, the symptoms do not appear immediately after epidural block, but they occur tens of minutes later. Therefore, if an outpatient has the block and is not observed sufficiently, it may cause a risky situation. Fortunately, the patient in this case was hospitalized and received proper treatment immediately because he was found in front of the elevator near his room where there were other patients and guardians. However, if he had been an outpatient, the treatment may have been delayed, resulting in greater risk.
In this case, about 35 minutes after the local anesthetic injection, the patient suddenly showed symptoms of apnea, unconsciousness, hypotension and bradycardia. Also, with the brain CT scan conducted after the patient's vital signs were stabilized, we diagnosed pneumocephalus. The patient may develop delayed symptoms for several reasons. First, we can think of the case in which an epidural block is not done but subdural injection is conducted. Uncommonly, subdural injection also might be done after an epidural block. If injected subdurally, even a small amount of local anesthetic might cause symptoms. If subdural injection were suspected, the onset of anesthetic effect is slower than epidural block and a high level of anesthesia may occur 15 to 35 minutes after local anesthetic injection [1]. Moreover, with a wide-ranging sympathetic block, hypotension may occur, causing apnea, respiratory distress, or unconsciousness [2]. This case may have fit that description, but because we did not perform C-arm guided block, we were not sure if a subdural block was done or not. Second, vasovagal syncope could have occurred while the patient was walking to his room. There are many causes of vasovagal syncope, and the exact mechanism is not known. However, the imbalance of sympathetic and parasympathetic tone may cause bradycardia and/or hypotension [3]. Therefore, there is a good possibility that vasovagal syncope caused this patient's symptoms. Third, we can also think of this possibility. Since CSF was not aspirated, if epidural block was conducted, extensive sympathetic block was done, too. And the patient did not show symptoms while lying on the bed but lost consciousness when standing up on account of the serious reduction of BP.
We also must consider pneumocephalus as a cause. In this case, pneumocephalus was diagnosed from the brain CT scanning to check if there was intracranial hemorrhage. Brain and spinal cords are covered with three membranes called meninges. The outer layer is referred to as the dura mater, the middle layer as the arachnoid mater, and lastly, the inner layer as the pia mater. Subdural space refers to the space between the dura mater and the arachnoid mater, and it leads to the cranium from the lumbar spine. Subdural space is widest in the cervical region and smallest in the lumbar region, and it is filled with a small volume of serous fluid. Although subdural space is not directly connected to the subarachnoid space, nerve roots and dorsal root ganglia come out laterally, and unlike epidural space, subdural space is connected to intracranial space [4]. Also, subdural space is connected to the floor of the third ventricle in the cranial cavity from the lower border of the second sacral vertebra [5]. Therefore, it can be assumed that in this patient, pneumocephaus may have resulted from air entering the subdural space and flowing into the intracranium.
However, some say that subdural space is not potential space but a cleft formed in the meninges due to trauma or tissue damage [6]. Reina et al. [6] describes the ultrastructure of subdural space. They say that there is a cellular junction called the dura-arachnoid interface between the laminar arachnoid portion and the inner surface of the dura, and this space consists of neurothelial cells surrounded by amorphous substances. Therefore, although there is no subdural space in the untraumatized tissues, neurothelial cells become destroyed by mechanical force, air, or fluid injection to form fissures in the amorphous substances of the interface. Fissures expand to a weaker area, and at this time, they expand laterally to the area with more amorphous substances [6]. Therefore, the pneumocephalus may have resulted from air coming to the subdural space and then flowing into the subarachnoid through the weak region of the dura.
When falling, the patient was reported to have injured the right temporal area. We could not find any skull fracture on the brain or facial bone CT scan. However, it is possible that there was a fracture on a region we couldn't see from the CT scan, so we think it possible that air may have entered there from outside.
We used about 8 cc of air when conducting the lumbar epidural block. Thus, we cannot exclude the possibility that air may have entered there through dural puncture site even though CSF aspiration was not done.
Pneumocephalus means that there is air in the cranial cavity. It may result from trauma, craniofacial surgery, spinal surgery, lumbar puncture, epidural steroid injection, or Valsalva's maneuver [7-9]. In case of postdural puncture headache (PDPH), the pain is relieved when the patient lies down, but it gets worse when he sits or stands. Sometimes it appears right after the dural puncture, but it occurs more frequently 24 to 48 hours later [10]. Pneumocephalus is often not accompanied by symptoms, but the most common symptom is headache. It is known that the cause of the headache is the rapid brain movement resulted from air injection and meningeal irritation. Posture change may worsen the headache, but it does not get better even if the patient lies down. If a cranial nerve were compressed by air, the patient might show neurologic symptoms such as visual impairment, hearing defect, tinnitus, and mydriasis [11,12]. This patient complained of severe occipital headache and severe facial itchiness, but the cause of itchiness could not be found.
100% oxygen should be administered immediately in the supine position once pneumocephalus is diagnosed [13]. This is to accelerate the absorption of intracranial air by increasing the diffusion gradient for nitrogen between the air collection and the surrounding cerebral tissue [14]. Also, we can administer aggressive hydration, caffeine, or analgesics to relieve headache [15]. For this patient, we used midazolam that has nothing to do with the treatment of pneumocephalus. Because the patient suffered from serious facial itchiness, and it was out of control, we used it for sedation.
Generally, in pneumocephalus, air is absorbed within 24 hours, and patients usually recover without any special neurologic abnormalities. Occasionally, however, persistent headache, lethargy, confusion, slow arousal, hemiparesis, or hemiplegia may also occur, and if tension pneumocephalus causes neurologic symptoms from the mass effect like a brain tumor of a large volume of air, a neurosurgical emergency treatment may be required. Thus, when there is any possibility of pneumocephalus, accurate evaluation is mandatory [16,17].
The way to minimize the possibility of pneumocephalus when performing epidural block with the loss of resistance technique is to use normal saline instead of air [18,19]. However, many pain physicians still prefer air. When air is used, they should try to minimize the amount of air to be used to minimize the risk of pneumocephalus. For epidural block, in order to reduce complication such as pneumocephalus, instead of using blind technique it would be more effective to us fluoroscopy. As such, it is important to understand the spread pattern of contrast media in epidural, subdural, and subarachnoid space. In addition, after epidural block, it is essential to check the vital sign of patients and ask them whether they are suffering from headache, dizziness, and motor weakness, among others. Make a thorough observation and if necessary take appropriate measures.
In conclusion, pneumocephalus is not a frequent complication, but once it occurs, it raises the risk of headache or other neurologic complications, so we should perform epidural blocks very carefully. In particular, when dural puncture is done, there is a possibility of pneumocephalus, so the patient should be monitored carefully after the procedure.
Go to : 
References
1. Collier CB. Accidental subdural injection during attempted lumbar epidural block may present as a failed or inadequate block: radiographic evidence. Reg Anesth Pain Med. 2004; 29:45–51. PMID: 14727278.

2. Abouleish E, Goldstein M. Migration of an extradural catheter into the subdural space. A case report. Br J Anaesth. 1986; 58:1194–1197. PMID: 3768232.
3. Bat T, Collins KK, Schaffer MS. Syncope during exercise: just another benign vasovagal event? Curr Opin Pediatr. 2011; 23:573–575. PMID: 21743327.
4. Ajar AH, Rathmell JP, Mukherji SK. The subdural compartment. Reg Anesth Pain Med. 2002; 27:72–76. PMID: 11799508.

5. Collier CB. Why obstetric epidurals fail: a study of epidurograms. Int J Obstet Anesth. 1996; 5:19–31. PMID: 15321378.

6. Reina MA, De Leon Casasola O, López A, De Andrés JA, Mora M, Fernández A. The origin of the spinal subdural space: ultrastructure findings. Anesth Analg. 2002; 94:991–995. PMID: 11916810.

7. Ozturk E, Kantarci M, Karaman K, Basekim CC, Kizilkaya E. Diffuse pneumocephalus associated with infratentorial and supratentorial hemorrhages as a complication of spinal surgery. Acta Radiol. 2006; 47:497–500. PMID: 16796314.

8. Clevens RA, Marentette LJ, Esclamado RM, Wolf GT, Ross DA. Incidence and management of tension pneumocephalus after anterior craniofacial resection: case reports and review of the literature. Otolaryngol Head Neck Surg. 1999; 120:579–583. PMID: 10187965.

9. Yoon SJ, Oh GS, Lee SJ, Lee BR, Chun JU, Yu IK. Pneumocephalus in patients with orthostatic headache. J Clin Neurol. 2008; 4:89–93. PMID: 19513309.

10. Benzon HT, Linde HW, Molloy RE, Brunner EA. Postdural puncture headache in patients with chronic pain. Anesth Analg. 1980; 59:772–774. PMID: 6448553.

11. Katz JA, Lukin R, Bridenbaugh PO, Gunzenhauser L. Subdural intracranial air: an unusual cause of headache after epidural steroid injection. Anesthesiology. 1991; 74:615–618. PMID: 1825771.
12. Nafiu OO, Urquhart JC. Pneumocephalus with headache complicating labour epidural analgesia: should we still be using air? Int J Obstet Anesth. 2006; 15:237–239. PMID: 16798452.

13. Kim YJ, Baik HJ, Kim JH, Jun JH. Pneumocephalus developed during epidural anesthesia for combined spinal-epidural anesthesia. Korean J Pain. 2009; 22:163–166.

14. Dexter F, Reasoner DK. Theoretical assessment of normobaric oxygen therapy to treat pneumocephalus. Anesthesiology. 1996; 84:442–447. PMID: 8602677.

15. Laviola S, Kirvelä M, Spoto MR, Tschuor S, Alon E. Pneumocephalus with intense headache and unilateral pupillary dilatation after accidental dural puncture during epidural anesthesia for cesarean section. Anesth Analg. 1999; 88:582–583. PMID: 10072010.

16. Katz Y, Markovits R, Rosenberg B. Pneumoencephalos after inadvertent intrathecal air injection during epidural block. Anesthesiology. 1990; 73:1277–1279. PMID: 2248409.

17. Black PM, Davis JM, Kjellberg RN, Davis KR. Tension pneumocephalus of the cranial subdural space: a case report. Neurosurgery. 1979; 5:368–370. PMID: 503299.
18. van den Berg AA, Nguyen L, von-Maszewski M, Hoefer H. Unexplained fitting in patients with post-dural puncture headache. Risk of iatrogenic pneumocephalus with air rationalizes use of loss of resistance to saline. Br J Anaesth. 2003; 90:810–811. PMID: 12765902.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download