This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Curcumin has been reported to have anti-inflammatory, antioxidant, antiviral, antifungal, antitumor, and antinociceptive activity when administered systemically. We investigated the analgesic efficacy of intrathecal curcumin in a rat model of inflammatory pain.
Methods
Male Sprague Dawley rats were prepared for intrathecal catheterization. Pain was evoked by injection of formalin solution (5%, 50 µl) into the hind paw. Curcumin doses of 62.5, 125, 250, and 500 µg were delivered through an intrathecal catheter to examine the flinching responses. The ED50 values (half-maximal effective dose) with 95% confidence intervals of curcumin for both phases of the formalin test were calculated from the dose-response lines fitted by least-squares linear regression on a log scale.
Results
In rats with intrathecal administration of curcumin, the flinching responses were significantly decreased in both phases. The slope of the regression line was significantly different from zero only in phase 2, and the ED50 value (95% confidence interval) of curcumin was 511.4 µg (23.5-1126.5). There was no apparent abnormal behavior following the administration of curcumin.
Conclusions
Intrathecal administration of curcumin decreased inflammatory pain in rats, and further investigation to elucidate the precise mechanism of spinal action of curcumin is warranted.
Go to :

Keywords: antinociception, curcumin, formalin test, spinal cord
INTRODUCTION
Curcumin (1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a major bioactive component of turmeric, which has been used as a coloring agent in food. It has been used as an herbal medicine for centuries in India, China, Japan, and Korea. Recently, curcumin has received a great deal of attention for its anti-inflammatory, antioxidant, antiviral, antifungal, and antitumor actions [
1-
4]. In addition, curcumin has been reported to have antinociceptive activity when administered systemically. The analgesic effects of curcumin are thought to be exerted by suppression of nitrite, TNF-α, and capsaicin-induced TRPV1 activity, and through the descending noradrenergic and serotonergic systems, [
5-
7]. Furthermore, curcumin has been shown to exhibit anti-inflammatory activity by means of inhibition of a number of different mediators including phospholipase, lipoxygenase, cyclooxygenase-2, leukotrienes, thromboxane, prostaglandins, and nitric oxide [
1,
8-
10]. As increased spinal cord expression of these substances is associated with pain [
11-
13], we hypothesized that intrathecal administration of curcumin may also produce antinociception.
However, neither the effect of intrathecally administered curcumin nor its spinal mechanism of action has been investigated. Therefore, the purpose of this study was to evaluate the analgesic efficacy of intrathecal curcumin in a rat model of inflammatory pain.
Go to :

MATERIALS AND METHODS
The experimental protocol was approved by the Institutional Animal Care and Use Committee, Chonnam National University. Experiments were conducted using adult male Sprague Dawley rats weighing 250-300 g. The rats were housed in individual cages in a temperature-controlled room (22 ± 0.5℃) with a 12/12-h light/dark cycle. Food and water were freely available. Intrathecal catheterization was performed for drug administration as described previously [
14]. Briefly, the rats were anesthetized with sevoflurane under spontaneous respiration, and placed in a stereotaxic head holder. A polyethylene-10 catheter was inserted through an incision in the atlantooccipital membrane. The catheter was advanced caudally 8.5 cm from the incision site to the lumbar enlargement of the spinal cord. The external end of the catheter was tunneled subcutaneously to exit at the top of the head and plugged with a piece of steel wire, and the skin was closed using 3-0 silk suture. The rats were closely monitored and euthanized through a volatile anesthetic overdose if motor abnormalities appeared. Normal rats were kept in individual cages, and a period of not less than 5 days was allowed for each rat to recover from intrathecal catheterization. Rats showing apparently normal behavior and weight gain were assigned to the experiment.
Curcumin ([1E,6E]-1,7-Bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione, Tocris Cookson Ltd., Bristol, UK) was dissolved in 100% dimethyl sulfoxide (DMSO). The drug was administered intrathecally in a 10 µl solution volume using a hand-driven gear-operated syringe pump, followed by an additional 10 µl of vehicle solution to flush the catheter. To examine the analgesic efficacy of curcumin in the formalin test, curcumin (62.5, 125, 250, 500 µg) was administered intrathecally to 5 rats in each group 10 min prior to the formalin test. Doses of curcumin were determined by the maximum solubility of the drug and for approximately equal spacing on the log scale.
On the day of the experiment, rats were acclimatized in a restraint cylinder for 15-20 min, and were randomly allocated into drug-treatment groups, each receiving one of the doses of curcumin, or the control group, receiving DMSO only. Each rat was used only once and the researcher who tested the drugs was blind to the drug administered to each animal. The formalin test was performed by subcutaneously injecting 50 µl of 5% formalin solution into the plantar surface of the left hind paw using a 30 gauge needle. The formalin injection evoked a characteristic spontaneous flinching behavior, and thus the pain behavior was quantified by periodically counting the number of flinches of the injected paw. The number of flinches was counted for 1 min periods from 1 to 2 min and 5 to 6 min, and every 5 min thereafter, up to 60 min. The observed responses appeared biphasically and were divided into phase 1 (0-9 min) and phase 2 (10-60 min). After the entire observation period, the rats were euthanized with an overdose of volatile anesthetic.
The behavioral effects of curcumin were evaluated in an additional group of rats (n = 5). Motor function was assessed for 60 min after intrathecal administration of 500 µg curcumin. The righting reflex was examined by placing the rat horizontally with its back on the table, which normally produces an immediate coordinated twisting of the body to achieve an upright position. The placing/stepping reflex was evoked by drawing the dorsum of either hind paw across the edge of the table, a stimulus which causes normal rats to try to put their paws forward into a position for walking. Pinna and corneal reflexes were evaluated by stimulating the ear canal or the cornea with a paper string. Normal rats spontaneously jerked their heads or blinked, respectively.
Data are expressed as mean ± SEM. The time-response data of the behavior evoked by formalin are presented as the number of flinches. The dose-response data are presented as a percentage of the control for each phase:
Dose-response data were analyzed using the Kruskal-Wallis test and Mann-Whitney U test. The ED
50 values (half-maximal effective dose) with 95% confidence intervals of curcumin for both phases of the formalin test were calculated from the dose-response lines fitted by least squares linear regression on a log scale [
15]. A test to determine whether the effect is dose-dependent was made from the F distribution as previously described [
15]. A value of
P < 0.05 was considered to indicate statistical significance.
Go to :

RESULTS
Subcutaneous injection of formalin into the hind paw produced a biphasic flinching response, with an early (phase 1) response lasting 5-10 min, and after a quiescent interval of 5-10 min, a subsequent late (phase 2) response up to 60 min.
Fig. 1 shows the time course and dose-response data of intrathecal curcumin, administered 10 min before formalin injection, in the formalin test. In the control group with intrathecal injection of DMSO, the total number of flinches was (mean ± SEM) 28 ± 5 during phase 1 and 220 ± 15 during phase 2.
Fig. 2A and 2B show the dose-response curves of intrathecal curcumin on flinching response during both phases of the formalin test. In rats with intrathecal administration of curcumin, the number of flinching responses was significantly decreased to 35-38% of the control group during phase 1 of the formalin test (
P < 0.05), but the extent of change was not statistically different over the range of administered doses (
P > 0.05, determined by Kruskal-Wallis test,
Fig. 2A). During phase 2, intrathecal delivery of curcumin suppressed the flinching response up to 49% of control, and the extent of change was statistically different over the range of doses (
P < 0.05, determined by Kruskal-Wallis test,
Fig. 2B).
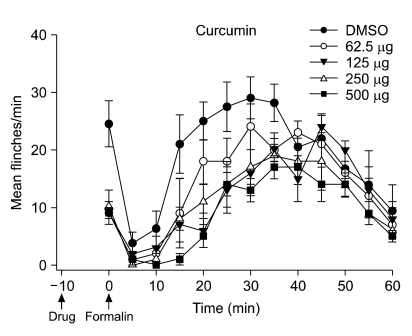 | Fig. 1Time course of intrathecal curcumin on flinching response during the formalin test. Curcumin was administered 10 min before the formalin injection. Data are presented as the mean of the number of flinches ± SEM of 5 rats at each time point. 
|
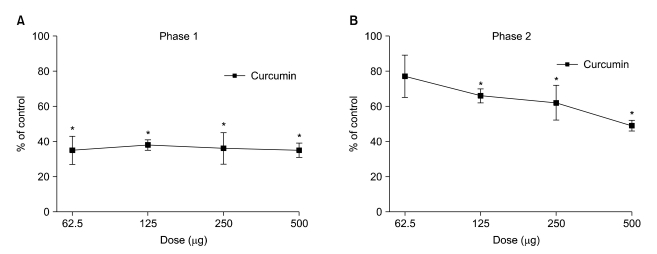 | Fig. 2Dose-response curves of intrathecal curcumin on flinching response during phase 1 (A) and phase 2 (B) in the formalin test. Curcumin was administered 10 min before the formalin injection. Data are presented as the percentage of control. Each line represents mean ± SEM of 5 rats. Compared with control, *P < 0.05. 
|
The calculated F values for linear regression of analgesia on log dose of curcumin were 0.036 and 5.902, for phases 1 and 2, respectively. The F value of phase 2 exceeds the tabular F = 4.41 based on the degrees of freedom for this study (1, 18) for P < 0.05. These data indicate that the slope of the regression line was significantly different from zero only in phase 2 and that the curcumin-produced analgesia was dose-dependent in phase 2 but not phase 1 of the formalin test. Consequently the ED50 value (95% confidence interval) of curcumin was calculated only for phase 2 and was 511.4 µg (23.5-1,126.5).
There was no apparent abnormal behavior in the rats following the administration of curcumin.
Go to :

DISCUSSION
Formalin-induced nociception consists of two different nociceptive states, and distinct mechanisms underlie the two phases of behavioral response. Acute nociception (phase 1) is followed by the facilitated state (phase 2). The phase 1 response appears to result from the immediate and intensive increase in activity of the primary afferent fibers induced by formalin, reflecting an acute pain. On the other hand, the phase 2 response may result from the activation of wide dynamic range neurons in the dorsal horn, with a continuous low level of activity in the primary afferent fibers [
16]. Therefore, phase 2 represents a facilitated state which appears to be a prominent and intensified pain state in spite of a decreased level of afferent input. This pain model has been utilized as a tool for observing the effects of various antinociceptive agents on these two types of pain at once.
In the present study, we evaluated the analgesic effect of curcumin at the spinal level in a rat model of inflammatory pain. Intrathecally administered curcumin reduced the flinching response evoked by formalin injection during both phases. Intrathecal curcumin also produced no behavioral abnormalities. These findings suggest that curcumin possesses a spinal mechanism of action, and present the possibility of curcumin as a novel analgesic agent to be delivered in the spinal cord.
Previous reports have demonstrated analgesic effects of systemically administered curcumin in rodent models of diabetic neuropathic pain [
5], formalin-induced orofacial pain [
17], capsaicin-induced thermal hyperalgesia [
6], and chronic constriction injury-induced neuropathic pain [
7]. The mechanisms underlying the analgesic action of systemically administered curcumin have been suggested to be associated with suppression of brain nitrite and serum tumor necrosis factor α levels [
5], antagonistic effects on the transient receptor potential vanilloid 1 channel [
6], and the descending monoamine system coupled with opioid receptors [
7]. Although the spinal analgesic effect of curcumin has not been studied, the results of the study of Zhao et al. [
7] are consistent with the current study. Intrathecal but not intracerebroventricular injection of adrenergic or serotonergic antagonists blocked the antinociceptive effect of curcumin [
7]. As curcumin can pass through the blood-brain barrier [
18], systemically administered curcumin could have enhanced spinal serotonergic or adrenergic transmission, which might also contribute to the antinociception produced by intrathecal administration in the current study.
The spinal cord represents a novel site of investigation for pain research, positioned optimally for regulation of pain both in respect to convenient external access for drug delivery and relative isolation from other parts of the central nervous system. Application of spinal delivery of analgesic agents in animal studies has contributed to advances in understanding of the basic science of pain and the analgesic mechanisms of experimental drugs [
19]. Furthermore, the establishment of intrathecal drug administration methods in humans has provided a new pain relief option for patients suffering from chronic intractable pain refractory to systemically administered analgesics [
20]. As the spinal cord neurons are not known to regenerate, development of novel compounds for spinal delivery must pass particularly strict screening tests before trials in humans [
19]. Although curcumin has been shown to be safe in general, it has been demonstrated to cause some gastric irritation in humans [
21] and hepatotoxicity in rodents at high doses [
22,
23]. The findings in the current study may therefore raise the possibility of curcumin as a novel analgesic for spinal delivery, reducing the adverse side effects of systemic administration and thus providing therapeutic benefit in some patients, and further investigation is warranted to elucidate its precise mechanism of spinal action.
There are some limitations to this study. The mechanisms of the observed curcumin-produced analgesia were not elucidated and remain to be further examined. In addition, the differential effects of intrathecal curcumin on the acute and facilitated states should be explored in a future study.
In conclusion, intrathecal administration of curcumin decreased inflammatory pain in rats, and further investigation is warranted to elucidate the precise mechanism of spinal action of curcumin.
Go to :

ACKNOWLEDGEMENTS
This study was supported by a grant (CRI10016-1) of the Chonnam National University Hospital Research Institute of Clinical Medicine.
Go to :

References
1. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003; 9:161–168. PMID:
12676044.

2. Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991; 51:813–819. PMID:
1899046.
3. Oetari S, Sudibyo M, Commandeur JN, Samhoedi R, Vermeulen NP. Effects of curcumin on cytochrome P450 and glutathione S-transferase activities in rat liver. Biochem Pharmacol. 1996; 51:39–45. PMID:
8534266.

4. Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995; 55:259–266. PMID:
7812955.
5. Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006; 536:256–261. PMID:
16584726.

6. Yeon KY, Kim SA, Kim YH, Lee MK, Ahn DK, Kim HJ, et al. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res. 2010; 89:170–174. PMID:
20040737.

7. Zhao X, Xu Y, Zhao Q, Chen CR, Liu AM, Huang ZL. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: Descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2011; [in press].

8. Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol Immunotoxicol. 2003; 25:213–224. PMID:
12784914.

9. Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC. Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol Mol Hematol. 1997-1998; 11:49–62. PMID:
9439980.
10. Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001; 172:111–118. PMID:
11566484.

11. Yamamoto T, Nozaki-Taguchi N. The role of cyclooxygenase-1 and -2 in the rat formalin test. Anesth Analg. 2002; 94:962–967. PMID:
11916805.

12. Coderre TJ, Abbott FV, Sawynok J. Schmidt RF, Willis WD, editors. The formalin test. Encyclopedia of pain. 2007. New York: Springer;p. 795–799.
13. Sommer C, Sorkin LS. Schmidt RF, Willis WD, editors. Cytokines as targets in the treatment of neuropathic pain. Encyclopedia of pain. 2007. New York: Springer;p. 518–520.

14. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036. PMID:
14677603.

15. Tallarida RJ. Drug synergism and dose-effect data analysis. 2000. Boca Raton (FL): Chapman and Hall/CRC.
16. Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996; 64:345–355. PMID:
8740613.

17. Mittal N, Joshi R, Hota D, Chakrabarti A. Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytother Res. 2009; 23:507–512. PMID:
19051211.

18. Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011; 416:331–338. PMID:
21729743.

19. Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003; 55:1007–1041. PMID:
12935942.

20. Fitzgibbon DR. Fishman SM, Ballantyne JC, Rathmell JP, editors. Cancer pain: principles of management and pharmacotherapy. Bonica's management of pain. 2009. 4th ed. Philadelphia: Lippincott Williams & Wilkins;p. 583–603.
21. Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986; 24:651–654. PMID:
3546166.
22. Deshpande SS, Lalitha VS, Ingle AD, Raste AS, Gadre SG, Maru GB. Subchronic oral toxicity of turmeric and ethanolic turmeric extract in female mice and rats. Toxicol Lett. 1998; 95:183–193. PMID:
9704820.

23. Kandarkar SV, Sawant SS, Ingle AD, Deshpande SS, Maru GB. Subchronic oral hepatotoxicity of turmeric in mice--histopathological and ultrastructural studies. Indian J Exp Biol. 1998; 36:675–679. PMID:
9782784.
Go to :








 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download