This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Pregabalin has been shown to have analgesic effect in acute pain models. The primary objective was to examine the efficacy a single dose of pregabalin, would have on morphine consumption following lumbar discectomy.
Methods
With ethical approval a randomized, placebo-controlled prospective trial was undertaken in 32 patients (ASA I-II, 18-65 years) with radicular low back pain for > 3 months undergoing elective lumbar discectomy. Patients received either oral pregabalin 300 mg (PG Group) or placebo (C Group) one hour before surgery. Pain intensity, the accumulative morphine consumption and adverse effects were recorded for 24 hours following surgery. Functional, psychological and quantitative sensory testing were also assessed.
Results
Fourteen patients out of the 32 recruited were randomized to receive pregabalin. Morphine consumption was reduced (absolute difference of 42.3%) between groups with medium effect size. (Mann-Whitney; U = 52.5, z-score= 2.84, P = 0.004, r = 0.14). This was not associated with a significant difference in the incidence of adverse effects between the two groups. The median pain intensity (VAS) on movement was not significantly different between groups.
Conclusions
A single pre-operative dose of pregabalin (300 mg) did not result in a reduction in pain intensity compared to placebo in this patient cohort but the significant reduction in morphine consumption suggests that a fixed peri-operative dosing regime warrants investigation.
Go to :

Keywords: morphine consumption, post-operative pain, pregabalin
INTRODUCTION
Despite major improvements in our understanding of acute pain physiology over the past decade, approximately 80% of patients undergoing surgical procedures experience postoperative pain [
1]. Acute postoperative pain is recognized as a predictor of persistent post surgical pain and between 5 and 50% of patients reported persistent post surgical pain after a variety of common procedures [
2]. Recent advances in the pathophysiology of pain have suggested that it is possible to prevent or attenuate the central neural hyperexcitability that contributes to enhanced postoperative pain [
3]. Although opioids are an important component of postoperative pain management, they are associated with side effects, and so, a multimodal analgesic approach has been recommended for the management of acute postoperative pain [
4].
Pregabalin has been shown to have a analgesic and opioid-sparing effect in the postoperative period [
5,
6]. However the analgesic efficacy of pregabalin (300 mg) given pre-operatively in a cohort of patients known to suffer from chronic pain is unknown.
The pre-study hypothesis is that a single pre-operative dose of pregabalin (300 mg) would result in a significant reduction in morphine consumption with a parallel reduction in acute pain intensity following lumbar discectomy.
Go to :

MATERIALS AND METHODS
1. Patient population
With institutional ethical committee approval and having obtained informed written consent a prospective study of all ASA I-II patients, 18-65 years old, undergoing elective lumbar discectomy at a single institute were included. Patients were considered for inclusion if they had an intervertebral disc herniation confirmed on magnetic resonance imaging, and persistent symptoms despite non-operative treatment for at least 12 weeks. Specific inclusion criteria were the presence of radicular pain and/ or low back pain and evidence of nerve-root irritation with a positive nerve-root tension sign (straight leg raise positive between 30° and 70° or positive femoral tension sign) or a corresponding neurological deficit (dermatomal distribution of pain, asymmetrical depressed reflex, decreased sensation in a dermatomal distribution, or weakness in a myotomal distribution). Lumbar disc protrusion was confirmed using pre-operative magnetic resonance imaging as reported by independent radiologists. Patients requiring lumbar discectomy from L1/L2 to L5/S1 were eligible for inclusion included provided that only one of the herniations was judged to be symptomatic.
Exclusion criteria included previous spinal surgery, cauda equina syndrome, known spinal or genetic abnormalities, pregnancy, other chronic pain states, vertebral fractures, spinal infection or tumour, inflammatory spondyloarthropathy, pre-operative analgesia management that included gabapentin, pregabalin or opioids in the two weeks prior to surgery. The use of paracetamol (1 gram), every 6 hours, was permitted.
2. Intervention and blinding
Patients were randomly allocated, using a computer generated random numbers table, into one of two groups to receive (i) pregabalin 300 mg orally (PG) or (ii) a placebo (C) one hour before surgery. Placebo drugs consisted of matching sugar capsules and were prepared by the hospital pharmacy. Study medications, contained in sealed envelopes, were labelled with the name of the project and the allocated random number. Telephone communication between the operating theatre and the hospital ward, ensured that the prescribed "pre-medication" was administrated on time. At no point was the patient, the investigators, or the healthcare staff aware of the group assignment until the study concluded.
3. Anaesthetic technique, analgesia & surgery
With standard hemodynamic monitors in place and after three minutes of pre-oxygenation anaesthesia was induced with fentanyl 1-2 ug/kg, propofol 2-4 ug/kg followed by vecuronium 0.8 ug/kg to facilitate intubation and controlled ventilation. Anaesthesia was maintained using sevoflurane (0.8-1.5%) in N20/O2 (70:30) and intermittent vecuronium as clinically indicated. During surgery each patient received a fentanyl intravenous bolus of 25 ug as required if the heart rate increased by 10% compared to the pre-induction baseline. Each patient received paracetamol (1 gram) and diclofenac (75 mg) intravenously before surgery commenced. Prior to skin closure the subcutaneous tissue was infiltrated with 10 mls of bupivacaine 2.5 mg/ml by the neurosurgeons. Ondansetron (4 mg, intravenously) was administrated for prophylaxis of nausea and vomiting in all cases.
A standardised open microscopic lumbar discectomy surgical technique, with examination of the affected nerve root was performed by one of three consultant neurosurgeons at our institute. The surgical and anaesthetic time (from induction to tracheal extubation) was recorded on the theatre computerised records.
Each patient was prescribed paracetamol (1 g, orally) every six hours for 24 hours and a patient controlled analgesia (PCA) as part of their post-operative pain management. This consisted of intravenous morphine (2 mg per bolus), with a six minute lock-out interval, without a continuous infusion. This was commenced in the post-operative care unit (PACU) and it was available to the patient for 24 hours post discharge from the PACU. If analgesia was inadequate, a nurse practitioner could over-ride the PCA protocol and administer additional intravenous morphine in increments of 1 mg in keeping with hospital policy. The total volume of morphine consumed was electronically recorded on the patient controlled analgesic device for the duration of the study. Additional doses of ondansetron, 4 mg intravenously, were administered as clinically indicated. Patients were discharged to the ward from the postoperative care unit, after approximately 20 minutes and only if they had a verbal analogue pain score < 2 out of ten and a sedation score of 1 out of five [
7] in keeping with local protocol.
4. Clinical outcomes
Pain intensity was recorded using a visual analogue scale (VAS), consisting of a 10 cm horizontal line with the two end-points labelled "no pain" and "worst pain ever" at six assessment time points: pre-operatively, on discharged from the PACU, 4, 8, 12 and 24 hours following surgery. The VAS scores and the incidence of adverse effects were independently recorded by a member of the acute pain team in keeping with hospital protocol for the use of PCA morphine. Morphine consumption was electronically recorded on the PCA device and downloaded after 24 hours. The incidence of adverse effects; nausea, somnolence, light-headedness, headache, dizziness, visual disturbances, and vomiting were recorded at the end of the 24 hour period and patients were asked to rate on a four point verbal scale (none, mild, moderate, severe) the presence and severity of each side effect if appropriate. A record of the number of patients who experienced nausea and/or vomiting, and the total quality of ondansteron administrated was kept.
The Short-form McGill pain questionnaire [
8] was used to assess pain quality and the Roland-Morris Questionnaire was completed by patients to assess physical disability due to low back pain [
9]. Psychological assessment included the Hospital anxiety and depression scale questionnaire [
10,
11] and pain coping strategies were investigated by the Pain Catastrophizing Scale [
12]. The assessments were distributed to the patients for self completion at their bedside the day before surgery. The researcher explained the instructions for each assessments/ questionnaires in turn and remained available to the patient until they were completed. Each patient received the assessments in the same order. A second assessment was completed by the patients 24 hours after surgery. On both occasions the assessments/questionnaires were checked for completeness while the patient was present. Following completion of the assessments/questionnaires the data was subsequently transferred to an electronic record (Microsoft Excel 2007) for analysis.
5. Neurophysiological assessments
Quantitative sensory testing was preformed pre-operatively and post-operatively by a single trained investigator. The pain perception threshold to transcutaneous constant current electrical stimulation was assessed in each patient lying supine in a warm quiet environment using a Dantec Keypoint Neurodiagnostic stimulator. Patients were unable to see the monitor, were not distracted during the testing and were given the identical instruction. Pain perception thresholds were recorded, five minutes apart using a 0.1 mA/sec ramping rate, and a standardized technique in the forearm (C8-T1 dermatome) contralateral to the nerve root involved, and in the affected dermatome of the affected and contralateral lower limbs. If two thresholds values differed by > 20% between runs, testing was repeated until three consecutive thresholds were recorded each within 20% of both.
6. Data and power analysis
Based on previous preliminary results from our department, the anticipated morphine requirement in the first 24 hours following lumbar discectomy was 12 mg (Standard deviation = 5 mg). We considered a 40% reduction in morphine consumption to be clinically relevant then, with an alpha = 0.05 and beta = 0.20, at least 18 patients are required to be enrolled in each group to allow for loss to follow up.
The method of analysed was decided prospectively and included unpaired Student t-tests, ANOVA and Fisher Exact tests with bonferroni correction where appropriate. The VAS pain scores were analysed using Krushal-Wallis and Mann-Whitney U-tests; the incidences of side-effects were analysed with Fisher's exact test. The Microsoft Excel (2007) package was used for statistical analysis.
P = 0.05 was considered significant. Data was presented in keeping with CONSORT 2010 guidelines regarding randomised trials (
www.consort-statement.org).
Go to :

RESULTS
Thirty-six consecutive patients who fulfilled the inclusion criteria were considered for the study. Following randomization two patients were subsequently excluded because they received opioid analgesia before surgery. Another two patients chose not to continue and were with drawn before they received their assigned "pre-medication". Of the resulting thirty-two patients, 14 were randomly assigned to the PG group and 18 to the C group (
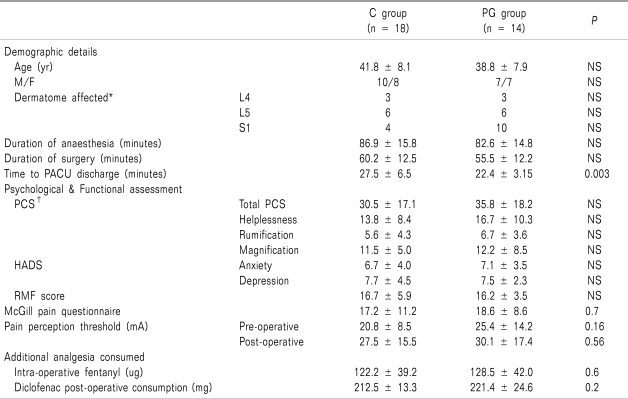
Fig. 1). The baseline demographic and clinical characteristics of each group are shown in
Table 1. Both groups were homogeneous in terms of pre-operative pain intensity, paracetamol consumption, duration of pain, psychological and functional scores and pre-operative neurophysiological assessments (
Table 1).
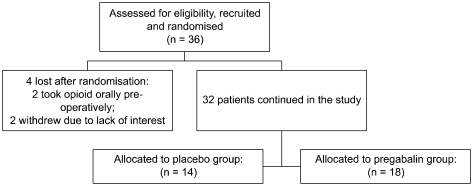 | Fig. 1Flow diagram of patient distribution. 
|
Table 1
The Demographic, Psychological, Functional, Pain Perception Thresholds and Analgesic Requirements for the Placebo (C) and Pregabalin (PG) Groups

The accumulative morphine consumption in the postoperative 24 hours was 2.5 times greater in the C group compared to the PG group (187 mg v 74 mg). Mann-Whitney analysis shows a significant difference in median morphine consumption between the C group and the PG group with medium effect size. (U = 52.5, z-score = 2.84,
P = 0.004, r = 0.14) (
Fig. 2). This represents an absolute difference of 42.3% in morphine consumption during the study.
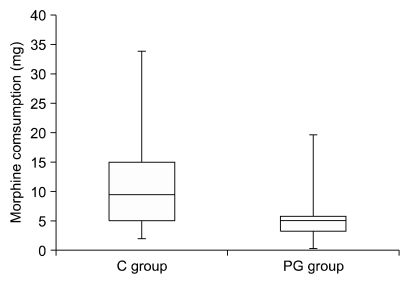 | Fig. 2This figure represents accumulative 24 hour morphine consumption (mg) in the 24 hours following lumbar discectomy. C group: the placebo, PG group: pregabalin group. Boxes show interquartile ranges and the bars are the 10th and 90th percentiles. Mann-Whitney analysis shows a significant difference in median morphine consumption between the C group and the PG group with medium effect size (r) (U = 52.5, z-score = 2.84, P = 0.004, r = 0.14). 
|
The median pain intensity (VAS) on movement was significantly reduced following surgery in both the C group [Kruskal-Wallis test; H(5) = 52.9,
P < 0.001] and in the PG group [H(4) = 35.8,
P = 0.001] (
Table 2). No significant difference in pain intensity between the groups was found pre- or post-operatively (
Table 3).
Fig. 3 shows the box plot for the median pain scores (25
th-75
th percentiles) in the 24 hours following surgery. The absolute difference in VAS scores over the 24 hour study period is shown in
Table 2.
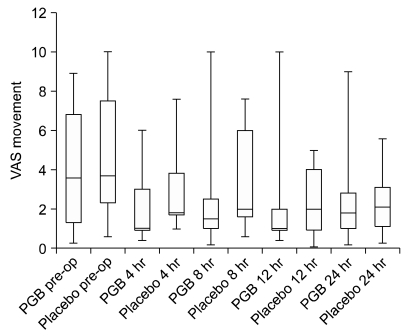 | Fig. 3Peri-operative pain intensity score (VAS) on movement are showing. Boxes show interquartile ranges and the bars are the 10th and 90th percentiles. Krushall-Wallis testing showed a significant reduction in pain scores within both the PGB group (H(4) = 35.8, P = 0.001) and the placebo group (H(4) = 52.9, P < 0.001). Post hoc Mann-Whitney analysis showed no significant difference between tow groups. 
|
Table 2
Pain Intensity (VAS) on Movement Pre- and Postoperatively following Lumbar Discectomy
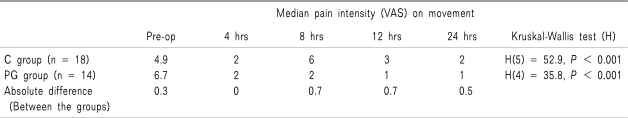

Table 3
Pre- and Post-operative Median Pain Intensity Scores


Post hoc analysis, using Mann Whitney tests and applying a bonferroni correction so all events were reported at a 0.0167 level of significance, showed that both the C group (Mann-Whitney U-test; U = 17.5, z-score = 4.64, P < 0.001, r = 0.7) and PG group (U = 14.0, P < 0.001) reported significantly less pain up to 4 hours following surgery. Both groups continued to show a reduction in the median pain scores 8 and 12 hours following surgery.
There was no difference in the diclofenac, paracetamol and fentanyl consumption in either group during the 24 hour peri-operative period (
Table 1). The most common side effect was somnolence, which was typically described as mild to moderate by 7 patients in C group (38.8%) and 8 patients in PG group (57%). Light headedness and dizziness were reported in our study but both were described as mild to moderate. No reduction in the incidence of adverse effects was identified in the study (
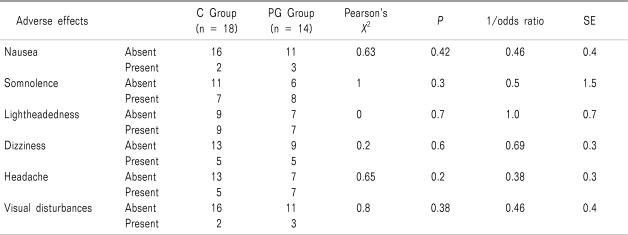
Table 4). Post-hoc analysis showed that when patients who did not report any adverse effects (absent) were compared to those who described the same symptom as mild/moderate or severe (present) there was no significant difference in the incidence of any of the adverse effects between either groups (
Table 5). Ondansetron (4 mg, intravenously), was administrated to two patients in both groups within the first 4 hours post-operative. The time to discharge from the post anaesthesia care unit (PACU) was slightly longer in the C group compared to the PG group (27.5 + 6.5 minutes vs 22.4 + 3.15 minutes,
P = 0.003) (
Table 1). All patients were ambulatory within 24 hours of surgery and were discharged from the hospital within 48 hours of admission.
Table 4
Adverse Effects in the First 24 Hours following Lumbar Discectomy
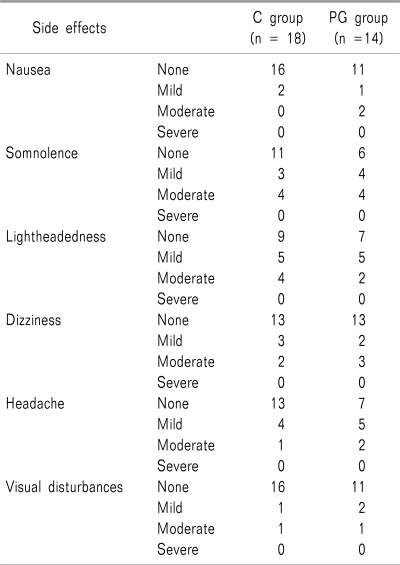

Table 5
Correlation Analysis Between Groups for the Incidence of Post-operative Adverse Effects following Lumbar Discectomy

Go to :

DISCUSSION
The key findings of this study are firstly, there was a 43.2% reduction in the total opioid consumption in the early post operative period; however, the opioid sparing effect was not associated with a significant difference in the incidence of adverse effects between the two groups. Secondly, a single dose of pregabalin (300 mg) administrated pre-operatively does not offer any significant reduction in pain intensity following lumbar discectomy compared to placebo.
Pregabalin has been shown to reduce acute post operative pain intensity and analgesic requirements in patients undergoing elective surgery with no pre-operative pain profile [
5,
6,
13]. The cohort population in our study are known to suffer chronic pain and the expectation is that central sensitisation has been established. The proposed mechanism of action of pregabalin is to limit the short-duration wind-up component of central sensitization by binds to the pre-synaptic alpha-2-delta subunit of the voltage-gated calcium channels (α2δ-VGCC) which are widely distributed in the spinal cord and the brain [
14,
15]. The conformational changes induced by this binding inhibit abnormally intense neuronal activity by reducing the synaptic release of glutamate and other neurotransmitters. Experimental studies with animal models and healthy human volunteers have shown that pregabalin reduces nociceptive responses, particularly in conditions involving central sensitization [
15]. Our results suggest that a single dose of pregabalin does not inhibit neuronal activity sufficiently to reduce pain intensity. While we accept that a reduction in pain intensity would have been more clinically meaningful the ability of one administration of pregabalin to reduce morphine consumption by 43% warrants further investigation.
To interpret our results we have to consider that either the dose of pregabalin and the dosing regime used was inadequate or a combination of both. We chose to use a pre-operative pregabalin dose of 300 mg because (i) this dose demonstrated a significant analgesic effect in an acute pain model with an acceptable adverse profile [
5,
16]; and (ii) a peak plasma concentration can be reliably achieved with 300 mg of pregabalin within one hour due to the predictable and linear pharmacokinetic profile of pregabalin [
3,
17]. As all patients in our study received their pre-operative intervention at the appropriate time it is expected that the peak plasma concentration for pregabalin was achieved. Other studies have prescribed pregabalin peri-operatively [
18] but not at the dose we used; the use of fixed or flexible pregabalin dosing regimes warrants further investigation to assess the best clinical outcome.
Whether the use of an enriched enrolment study design would enhance the average benefit of the study drug over placebo and result in important differences in this cohort is presently unknown [
19].
We accept that as all patients were assessed for side effects in the 24 hours following a general anaesthesia it is possible that this may have masked any side effects due to the pregabalin treatment. Furthermore our study was not powered to investigate the side-effects of pregabalin per se and further study design should include this fact. We also recognise that we failed to observe any reduction in the incidence of side effects associated with morphine consumption and thereby limits the clinical impact of this study. This may have been related to; (i) the low morphine requirement post operatively; (ii) the use of a PCA delivery system which is designed to avoid such complications; and (iii) the study was not powered to identify opioid related adverse effects. Indeed, for these reasons using adverse effect as the basis for our power analysis in our clinical setting would have not been practical. However, the pattern and degree of adverse effects we report are similar to those reported in previous studies [
6]. No patient in our study withdrew due to adverse effects and no significant differences in adverse effects were observed between the placebo and pregabalin groups. The most commonly reported adverse effect associated with the use of pregabalin is sedation [
6]; we found no significant difference between the groups in this regard. Likewise the patient's ability to ambulate postoperatively or the duration of hospital stay was not significantly different.
The study design ensured that both groups were homogenous in all other aspects and it is reasonable to conclude that the reduction in morphine consumption was related to the administration of pregabalin.
In conclusion, compared to placebo, a single pre-operative dose of pregabalin (300 mg) did not result in a significant reduction in acute pain intensity following lumbar discectomy but the morphine consumption was reduced in the 24 hour post-operative period following lumbar discectomy. This morphine-sparing effect was not, however, associated with a reduced incidence of reported opioid induced side-effects. Further studies are required to examine the benefit of continued prescribing of pregabalin peri-operatively following lumbar discectomy and the impact that this might have on chronic post-surgical pain.
Go to :

ACKNOWLEDGEMENTS
Dr. D. Hegarty received departmental funding as a clinical pain research fellow when this research was undertaken as part of a PhD. submission to the University College, Cork. The research fund at the Department of Anaesthesia & Pain medicine at Cork University Hospital received supported from the Pfizers' company but this support was not directly or indirectly related to the subject matter of this study. The Pfizers' Company or its representatives had no input into the content, design or analysis of this study at any time. Therefore, there is no conflict of interest to be reported with regard to any commercial, non-commercial affiliations or consultancy work. This was presented in part as an abstract/poster presentation at the American Society of Anaesthetists ASM at Orlando, Florida, 2008.
Go to :

References
1. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003; 97:534–540. PMID:
12873949.

2. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006; 367:1618–1625. PMID:
16698416.

3. Buvanendran A, Kroin JS, Kari M, Tuman KJ. Can a single dose of 300 mg of pregabalin reach acute antihyperalgesic levels in the central nervous system? Reg Anesth Pain Med. 2010; 35:535–538. PMID:
20975469.

4. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002; 183:630–641. PMID:
12095591.

5. Hill CM, Balkenohl M, Thomas DW, Walker R, Mathé H, Murray G. Pregabalin in patients with postoperative dental pain. Eur J Pain. 2001; 5:119–124. PMID:
11465977.

6. Paech MJ, Goy R, Chua S, Scott K, Christmas T, Doherty DA. A randomized, placebo-controlled trial of preoperative oral pregabalin for postoperative pain relief after minor gynecological surgery. Anesth Analg. 2007; 105:1449–1453. PMID:
17959981.

7. Némethy M, Paroli L, Williams-Russo PG, Blanck TJ. Assessing sedation with regional anesthesia: inter-rater agreement on a modified Wilson sedation scale. Anesth Analg. 2002; 94:723–728. PMID:
11867405.

8. Melzack R. The McGill pain questionnaire: from description to measurement. Anesthesiology. 2005; 103:199–202. PMID:
15983473.
9. Ostelo RW, de Vet HC, Knol DL, van den Brandt PA. 24-item Roland-Morris disability questionnaire was preferred out of six functional status questionnaires for post-lumbar disc surgery. J Clin Epidemiol. 2004; 57:268–276. PMID:
15066687.

10. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–370. PMID:
6880820.

11. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002; 52:69–77. PMID:
11832252.
12. Sullivan MJ, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain. 2005; 113:310–315. PMID:
15661438.

13. Agarwal A, Gautam S, Gupta D, Agarwal S, Singh PK, Singh U. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth. 2008; 101:700–704. PMID:
18716003.

14. Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin--calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain. 2009; 142:13–16. PMID:
19128880.

15. Tuchman M, Barrett JA, Donevan S, Hedberg TG, Taylor CP. Central sensitization and Ca(V)α
2 δ ligands in chronic pain syndromes: pathologic processes and pharmacologic effect. J Pain. 2010; 11:1241–1249. PMID:
20472509.

16. Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008; 134:106–112. PMID:
17507163.

17. Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth. 2008; 101:535–541. PMID:
18653493.

18. Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010; 110:199–207. PMID:
19910619.

19. Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. Br J Clin Pharmacol. 2008; 66:266–275. PMID:
18489611.

Go to :









 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download