Abstract
Hyaluronidase is an enzyme that has temporary and reversible enzymatic effects on the matrix of connective tissue. When added to local anesthetics in pain treatments, it enhances their infiltration and dispersal into tissues. It is widely used in anesthesia for ocular, dental, and plastic surgery. Reports of drug hypersensitivity to hyaluronidase are rare and are usually confined to peribulbar or retrobulbar anesthesia during ophthalmic surgery. However, few reports exist on adverse drug reaction after epidural injection. We have observed two patients experiencing anaphylactic shock caused by hyaluronidase following epidural injection. Most of the patients with a hypersensitivity to hyaluronidase had one previous uneventful injection containing hyaluronidase, implying that sensitization had taken place. However, hypersensitivity occurring at the first administration is possible. A positive skin test can help establish the diagnosis. Although rare, the possibility of an allergic reaction to hyaluronidase should be considered even in patients with no known previous exposure.
Go to : 
Hyaluronidase is an enzyme that hydrolyzes the glucosaminic bond between hyaluronic acid, an intercellular substance, and connective tissue. It also increases penetration of interstitial drugs [1,2]. Thus, hyaluronidase is often used as an additive agent in many clinical areas, such as pain treatment, to improve the diffusion of drugs [3,4]. It is also mixed with local anesthetics in pain treatment to produce an enhanced anti-inflammatory and prolonged analgesic effect [5].
Although hyaluronidase has been in use for a long time, few reports exist on allergic reactions to it. Moreover, of those that exist, most concern allergic reactions that occur around the eyeballs following ophthalmic anesthesia (peibulbar or retrobulbar block) [2,6]. Recently, allergic reactions accompanying pain treatment have been reported as hyaluronidase has begun to be mixed with local anesthetics. However, anaphylactic shock following epidural injection is rarely reported [7].
We report here on cases of anaphylactic shock that occurred after the use of hyaluronidase during epidural block.
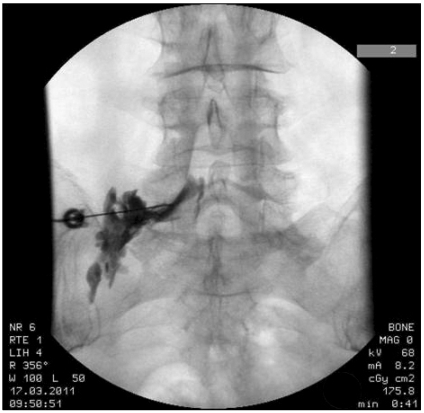
A 46-year old, female patient visited our clinic with the main complaints of back pain and numbness at the left lower limb, which had begun six months earlier. The patient stated that the pain had become severe since she had undergone discectomy at L4/5 for the back pain in April, 2010, which was one month before visiting our clinic. In the physical examination, the result of the straight leg raising test was 90°/60° and that of the Patrick test was negative. Numbness was found along the posterolateral aspect of the left lower limb, but t no weakening of muscular strength was observed. Tender points were present on the left gluteus medius and piriformis. The patient had no history of allergic reaction to any drugs, foods, or pollen. The MRI finding was "L4/5 bulging disc with annular tear, decompressed state and L5/S1 bulging disc." Under the diagnosis of post-laminectomy syndrome, a transforaminal epidural block was performed at the left L5 with 0.18% levobupivacaine 3 ml, triamcinolone 20 mg, and hyaluronidase 1,500 IU (Fig. 1). The procedure was performed under real-time fluoroscopic guidance, and the drugs were injected after insuring that there was no vascular uptake. The postoperative vital signs of the patient were stable with no allergic symptoms, such as skin rash. Since the back pain and the numbness at the left lower limb became severe again three months later, the procedure was repeated in the same manner with the same drugs and dosages. Immediately after the epidural injection, nausea, skin flare, generalized urticaria, respiratory distress, and hypotension (70/30 mmHg) occurred in the patient (Fig. 2). The symptoms were relieved after immediately injecting Ringer's lactate solution and performing an intravenous injection of ephedrine 5 mg, dexamethasone 5 mg, and chlorpheniramine maleate 4 mg.
Among the skin allergy tests, the skin prick test showed a negative finding for hyaluronidase and the contrast media, while the intradermal test showed a positive finding to hyaluronidase 15 IU/ml (1 : 100) with a wheal and flare at 8 mm. The intradermal test also showed a negative finding for the contrast media. The blood test showed that the total Ig E level was 86 IU/ml (0-183 IU/ml), which was normal.
A 35-year old, male patient visited our clinic with the main complaints of back pain and numbness in the right lower limb that had begun three years earlier. The patient had no history of allergic reaction or other specific findings. The patient had never undergone a nerve block that included hyaluronidase. However, the patient had had several bee venom injections as a form of treatment at an oriental medical clinic. We performed caudal epidural injection with 0.18% levobupivacaine 8 ml, triamcinolone 20 mg, and hyaluronidase 1,500 IU (Fig. 3). The procedure was performed under real-time fluoroscopic guidance. The drugs were injected after insuring that there was no vascular uptake. Immediately after the epidural injection, the patient experienced nausea and vomiting, skin flare, general urticaria, and hypotension (75/35 mmHg). The symptoms diminished immediately after injecting Ringer's lactate solution and giving an intravenous injection of ephedrine 5 mg, dexamethasone 5 mg, and chlorpheniramine maleate 4 mg.
Among the skin allergy tests, the skin prick test showed positive findings with a wheal and flare of 10 mm and 4 mm in diameters to hyaluronidase 1,500 IU/ml (1 : 1) and 150 IU/ml (1 : 10), respectively. Both the prick test and intradermal test showed a negative finding to the contrast media. The blood test showed that the total IgE level was 213 IU/ml (0-183 IU/ml), which is an elevated value.
Go to : 
Hyaluronidase is a synechotomic agent that decreases fibrosis and chronic inflammation that occurs after spinal surgery. Hyaluronidase is mixed with local anesthetics for pain treatment [8]. Schulze et al. [9] reported that hyaluronidase mixed with local anesthetics is effective for use as a local anesthesia because the activity of hyaluronidase is not decreased and, clinically, it reduces anesthetic dose and facilitate diffusion of local anesthetics.
Few reports exist concerning allergic reactions to hyaluronidase. The actual incidence is difficult to assess because weak allergic reactions are often recovered from spontaneously and, thus, may not be recognized. In most of the cases, allergic reactions to hyaluronidase occur when re-exposure to hyaluronidase is sensitized after previous exposure to it without any specific problems [10,11]. However, an allergic reaction to hyaluronidase may occur on the first exposure, even though this is rare [12]. In the first case reported here, the patient was sensitized to hyaluronidase after it was injected into the first epidural space. The second exposure caused an anaphylactic shock. The patient reported on in the second case had never undergone a nerve block that included hyaluronidase. However, this patient experienced anaphylactic shock after the first injection. The patient might have been exposed to hyaluronidase in bee venom, since he had undergone bee venom injection at an oriental medical clinic. The sensitization might have caused the anaphylactic shock. The venom of bees, red ants, scorpions, and hornets contains various protein allergens, including hyaluronidase, as 'spreading factors' which help spread the principal components of the venom. The hyaluronidases of the venom of these insects share similar sequence identities and structures [13].
The start of the allergic reaction may be acute, when it occurs during an operation after anesthesia, intermediate, when it occurs within a few hours, or delayed, when it occurs a few days or weeks later [10]. The main clinical symptoms of allergic reaction, following the use of anesthesia that contains hyaluronidase around the eyeballs during ophthalmic surgery, are local symptoms, such as edema around the eyeballs, flare, itching, pain, exopthalmosis, and limited motion of the extraocular muscle. The clinical symptoms of the two patients reported here occurred immediately after the injection of hyaluronidase. In these cases, anaphylactic shock accompanied general symptoms such as nausea and vomiting, general urticaria, and hypotension.
The type of allergic reaction caused by hyaluronidase has not been definitely identified. In many reported cases, the allergic reactions occurred during a second operation that was carried out after a patient had previously undergone injection of local anesthetics that included hyaluronidase, often in ophthalmic surgeries, such as a cataract surgery [12,14,15]. Skin allergy tests are important for the diagnosis and treatment of an allergy to hyaluronidase. An intradermal test is performed when the result of a prick test is not definite. A prick test gives a positive judgment if the wheal is greater than 4 mm, and the test is highly specific. An intradermal test gives a positive result if the wheal is greater than 8 mm. Such a test is highly sensitive. The intradermal test and prick test are commonly used to determine Type I (IgE mediated type and acute type) and Type IV (T-cell mediated type and delayed type) hypersensitive reactions [11,16]. Given that the allergic symptoms that appeared followed an exposure to hyaluronidase and given the results of the skin allergy tests, the allergic reactions to hyaluronidase may be considered Type I or Type IV hypersensitive reactions. For the patient in the first case, the prick test showed a negative result with respect to hyaluronidase and the contrast media. Thus, an intradermal test, which is more sensitive, was performed, which resulted in the positive response to the 1 : 100 diluted hyaluronidase of an 8 mm wheal. For the patient in the second case, the prick test showed positive reactions to hyaluronidase diluted at the ratios of 1 : 1 and 1 : 10, which appeared as wheals greater than 4 mm at two positions. Since the allergic reactions displayed by the patients in our cases occurred immediately after exposure to hyaluronidase and the subsequent skin allergy tests showed positive findings, the reactions are assumed to be Type I hypersensitive allergic reactions.
In many previous reports, hyaluronidase was either injected mixed with local anesthetics for ophthalmic surgeries, such as a cataract surgery, or it was intravenously injected as an additive during anticancer therapy [17,18]. The initial signs of allergic reactions are dependent on the injection pathways. Local angioedema or urticaria occurs if hyaluronidase is injected with local anesthetics, while anaphylactic shock, general urticaria, or respiratory distress occur in most patients if it is injected intravenously. The clinical symptoms of the allergic reactions are reduced within a few minutes using steroid treatment. Additional administration of an antihistamine improves the treatment and reduces the side effects of high steroid doses. In our cases, hyaluronidase was injected into the epidural space with local anesthetics for pain treatment. This caused a general anaphylactic shock which included severe hypotension. The symptoms were improved by using a steroid and an antihistamine as well as by injecting fluid and a pressor agent.
In conclusion, although allergic reactions to hyaluronidase are rare, the use of hyaluronidase should be avoided if a patient has a history of allergic reaction to hyaluronidase or if a skin allergy test shows a positive finding. Even though the clinical symptoms are weak and are often recovered from spontaneously, an anaphylactic reaction, which is accompanied by severe hypotension, may occur. An allergic reaction may occur upon the first administration of hyaluronidase even when a patient has had no known previous exposure.
Go to : 
References
1. Yocum RC, Kennard D, Heiner LS. Assessment and implication of the allergic sensitivity to a single dose of recombinant human hyaluronidase injection: a double-blind, placebo-controlled clinical trial. J Infus Nurs. 2007; 30:293–299. PMID: 17895809.

2. Feighery C, McCoy EP, Johnston PB, Armstrong DK. Delayed hypersensitivity to hyaluronidase (Hyalase) used during cataract surgery. Contact Dermatitis. 2007; 57:343. PMID: 17937752.

3. Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007; 80:1921–1943. PMID: 17408700.

5. Dunn AL, Heavner JE, Racz G, Day M. Hyaluronidase: a review of approved formulations, indications and off-label use in chronic pain management. Expert Opin Biol Ther. 2010; 10:127–131. PMID: 20420518.

6. Escolano F, Parés N, Gonzalez I, Castillo J, Valero A, Bartolomé B. Allergic reaction to hyaluronidase in cataract surgery. Eur J Anaesthesiol. 2005; 22:729–730. PMID: 16163925.

7. Kim JH, Choi GS, Ye YM, Nahm DH, Park HS. Acute urticaria caused by the injection of goat-derived hyaluronidase. Allergy Asthma Immunol Res. 2009; 1:48–50. PMID: 20224671.

8. Lee KJ, Han SG, Yoon SH, Kim JS, Lee YS. Nerve root block with corticosteroids, hyaluronidase, and local anesthetic in the failed back surgery syndrome (FBSS). J Korean Pain Soc. 1999; 12:191–194.
9. Schulze C, Bittorf T, Walzel H, Kundt G, Bader R, Mittelmeier W. Experimental evaluation of hyaluronidase activity in combination with specific drugs applied in clinical techniques of interventional pain management and local anaesthesia. Pain Physician. 2008; 11:877–883. PMID: 19057633.
10. Ahluwalia HS, Lukaris A, Lane CM. Delayed allergic reaction to hyaluronidase: a rare sequel to cataract surgery. Eye (Lond). 2003; 17:263–266. PMID: 12640426.

11. Kirby B, Butt A, Morrison AM, Beck MH. Type I allergic reaction to hyaluronidase during ophthalmic surgery. Contact Dermatitis. 2001; 44:52. PMID: 11156027.

12. Kempeneers A, Dralands L, Ceuppens J. Hyaluronidase induced orbital pseudotumor as complication of retrobulbar anesthesia. Bull Soc Belge Ophtalmol. 1992; 243:159–166. PMID: 1302146.
13. King TP, Spangfort MD. Structure and biology of stinging insect venom allergens. Int Arch Allergy Immunol. 2000; 123:99–106. PMID: 11060481.

14. Eberhart AH, Weiler CR, Erie JC. Angioedema related to the use of hyaluronidase in cataract surgery. Am J Ophthalmol. 2004; 138:142–143. PMID: 15234297.

15. Quhill F, Bowling B, Packard RB. Hyaluronidase allergy after peribulbar anesthesia with orbital inflammation. J Cataract Refract Surg. 2004; 30:916–917. PMID: 15093662.

16. Fisher MM, Bowey CJ. Intradermal compared with prick testing in the diagnosis of anaesthetic allergy. Br J Anaesth. 1997; 79:59–63. PMID: 9301390.

17. Leibovitch I, Tamblyn D, Casson R, Selva D. Allergic reaction to hyaluronidase: a rare cause of orbital inflammation after cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2006; 244:944–949. PMID: 16362314.

18. Szépfalusi Z, Nentwich I, Dobner M, Pillwein K, Urbanek R. IgE-mediated allergic reaction to hyaluronidase in paediatric oncological patients. Eur J Pediatr. 1997; 156:199–203. PMID: 9083759.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download