Abstract
Background
Interventional pain management (IPM) is a branch of medical science that deals with management of painful medical conditions using specially equipped X-ray machines and anatomical landmarks. Interventional physiatry is a branch of physical medicine and rehabilitation that treats painful conditions through intervention in peripheral joints, the spine, and soft tissues.
Methods
A cross-sectional study was conducted using three years of hospital records (2006 to 2008) from the Physical Medicine and Rehabilitation Department at Chittagong Medical College Hospital in Bangladesh, with a view toward highlighting current interventional pain practice in a tertiary medical college hospital.
Results
The maximum amount of intervention was done in degenerative peripheral joint disorders (600, 46.0%), followed by inflammatory joint diseases (300, 23.0%), soft tissue rheumatism (300, 23.0%), and radicular or referred lower back conditions (100, 8.0%). Of the peripheral joints, the knee was the most common site of intervention. Motor stimulation-guided intralesional injection of methylprednisolone into the piriformis muscle was given in 10 cases of piriformis syndrome refractory to both oral medications and therapeutic exercises. Soft tissue rheumatism of unknown etiology was most common in the form of adhesive capsulitis (90, 64.3%), and is discussed separately. Epidural steroid injection was practiced for various causes of lumbar radiculopathy, with the exception of infective discitis.
Conclusions
All procedures were performed using anatomical landmarks, as there were no facilities for the C-arm/diagnostic ultrasound required for accurate and safe intervention. A dedicated IPM setup should be a requirement in all PMR departments, to provide better pain management and to reduce the burden on other specialties.
Go to : 
According to the American Society of Interventional Pain Physicians (ASIPP), interventional pain management (IPM) is a 'discipline of medicine devoted to the diagnosis and treatment of pain related disorders.' IPM utilizes a multidisciplinary approach in which a team of health care professionals works together to provide a full range of treatments and services for patients suffering from chronic and/or acute pain. The goals of IPM are to relieve, reduce, or manage pain and improve a patient's overall quality of life through minimally invasive techniques specially designed to diagnose and treat painful conditions. The discipline also strives to help patients return to their everyday activities quickly and without heavy reliance on medications. The team members of IPM include a physiatrist (physical medicine and rehabilitation specialist), anesthesiologist, general surgeon, internist, and psychiatrist [1]. Interventional physiatry is a branch of physical medicine and rehabilitation that treats pain using precisely placed anti-inflammatory injections into the spine and pelvis, guided by specially equipped X-ray machines [2,3]. Interventional pain physicians may perform selective nerve root blocks, facet joint procedures, spinal cord stimulation, epidural injections, intrathecal pump placement, trigger point procedures, and vertebroplasty. They can also perform disc procedures such as discography, provocative discography, intradiscal electrothermaltherapy (IDET), etc. [3,4]. In 1998, the American Board of Physical Medicine and Rehabilitation joined the American Board of Anesthesiology in recognition of pain management as an interdisciplinary subspecialty [4,5]. In Bangladesh, however, the situation is completely different, with some limited spinal and peripheral joint procedures being performed by different specialists (physiatrists, orthopedists, anesthesiologists, neurologists, etc.) in their private practices. The subspecialty of interventional pain management does not exist, and there are no institutes devoted solely to IPM. In this article, we focus on the current practice of IPM in a tertiary medical college hospital in Bangladesh.
Go to : 
Patients who visited and received treatment through a pain clinic in the Department of Physical Medicine and Rehabilitation (PMR) at Chittagong Medical College Hospital over a period of 3 years (2006-2008) were enrolled in the study. Sources of patients were the outpatient department (PMR OPD), and the rheumatology follow up clinic, spondylarthropathy (SpA) clinic, and rheumatoid arthritis (RA) clinic, conducted in the department on separate days. Data were collected and recorded on a formulated data sheet containing each patient's particular and clinico-radiological information. To facilitate description, patients were categorized into the following 4 groups:
G-A: Degenerative peripheral joint disorders
G-B: Inflammatory rheumatological disorders
G-C: Non-infectious and non-inflammatory soft tissue rheumatism (STR)
G-D: Radicular and referred lower back pain (LBP)
Inflammatory joint disorders were classified according to the following clinical criteria: 1987 revised American College of Rheumatology (ACR) criteria for RA; modified New York criteria for ankylosing spondylitis (AS); and European Spondylarthropathy Study Group criteria for SpA [6-8]. Degenerative knee conditions were diagnosed according to clinical findings, with recommended clinical criteria used in some cases (ACR criteria for knee osteoarthritis, OA) [9]. Radiological corroboration was done where necessary. Pain in between the 12th rib and the inferior gluteal folds is known as LBP [10], and the common musculoskeletal sources of LBP are lumbar spondylosis, lumbar spinal stenosis, prolapsed lumbar intervertebral disc (PLID), myofascial pain syndrome (MPS), fibromyalgia (FMS), lumbar ligamentous sprain, piriformis syndrome, sacroiliac joint dysfunction, AS, vertebral body fracture, and spondylodiscitis. The term lumbar spinal stenosis is used to describe abnormal narrowing of the central part, lateral recesses, or intervertebral foramen of the lumbar spine to the point where the neural elements are compromised and signs or symptoms develop in the lower limbs. Common causes of lumbar spinal stenosis are lumbar spondylosis, lumbar spondylolisthesis, ligamentum flavum hypertrophy, facet joint arthropathy, PLID, and spondylodiscitis [10,11]. All of these clinical conditions except infective discitis were treated with interventional procedures in the pain clinic. Ligamentum flavum hypertrophy/facet hypertrophy was diagnosed with lumbar spine MRI. Soft tissue lesions can be due to trauma, overuse, infection, inflammation, or endocrinopathy, or may be idiopathic. In this paper, non-infectious and non-inflammatory soft tissue rheumatism are discussed separately.
The medications used for intervention were glucocorticoid derivatives (triamcinolone or methylprednisolone) and local injections of lidocaine [12]. Steroid doses ranged from 20-160 mg, with the maximum dosage (160 mg) given in lumbar interlaminar epidural injections and the minimum (20 mg) in intralesional (IL) procedures. During intraarticular (I/A) injection, large joints, medium-sized joints, and small joints received 40-80 mg, 20-40 mg, and 20 mg, respectively. Along with steroid injections, 1% lidocaine was used in a 2:1 ratio. The presence of effusion in the knee joint was determined by massage test (mild effusion), patellar tap (moderate effusion), and fluctuation test (huge effusion) [13]. Knee joint intervention was done with a lateral approach using a 22 G needle. In cases of OA of the knee, viscosupplement injection of sodium hyaluronate 20 mg/2 ml was used in some patients. Following knee intervention (both I/A steroid and viscosupplement), the joint was wrapped with a crepe bandage and movement was restricted for at least 24 hours. In the shoulder joint, both anterior and posterior approaches were employed. In the ankle joint, procedures wereperformed just medial to the tibialis anterior tendon. During lumbar epidural injections, 80-160 mg of steroid was placed in the epidural space using the LOR (loss of resistance) technique. These injections were usually performed at the L3-4 level, and sometimes at the L2-3 or L4-5 level due to difficulty with penetration at the L3-4 level. The epidural injections were performed in the prone position, with abdominal support to make the lumbar interlaminar space wider [14,15]. In the case of IL infiltration, the dose of steroid used ranged from 20-80 mg/infiltration, with the minimum in trigger finger (20 mg) and the maximum in piriformis syndrome (80 mg). During IL procedures, direct injection into the tendon sheath was avoided. In piriformis syndrome, the patient was placed in the prone position and the procedure was performed at the junction of the lateral one third and medial two thirds of the imaginary line between the greater trochanter and the intersection of the upper two thirds and lower one third of the sacroiliac joint, and the muscle was identified using anatomical landmarks, in some cases with motor stimulation [16]. Joint procedures were performed with methylprednisolone, whereas soft tissue intervention was done with triamcinolone acetonide except in cases of plantar fascia, in which methylprednisolone was preferred. Following intervention, antibiotic flucloxaciline (250-500 mg 4 times/day for 5 days) was prescribed. In immunocompromised patients, antifungal fluconazole (50 mg/day for 2 weeks) was added. Acute flare-ups of polyarticular or oligoarticular rheumatological conditions were managed with pulsed methylprednisolone (1,000 mg/day) in 100 ml of 5% dextrose aqua and treated for 3 consecutive days. Finally, a descriptive (cross-sectional) study was conducted using all medical records. The Bangladesh College of Physicians and Surgeons had reviewed and accepted all of these activities conducted in the department.
Go to : 
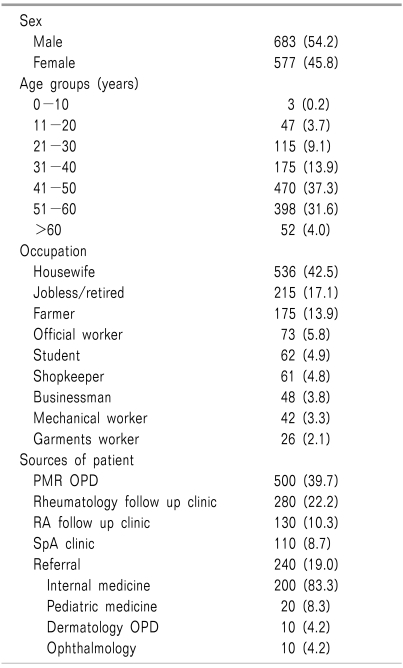
During the study period, a total of 45,842 patients were treated in the PMR OPD. Out of these patients, 1,260 (2.8%) with different articular and non-articular clinical conditions underwent intervention in the pain clinic. The patients' demographic profile is presented in Table 1. The greatest number of patients came from the PMR OPD (500, 39.7%), followed by the rheumatology follow up clinic (280, 22.2%), RA clinic (130, 10.3%), and SpA clinic (110, 8.7%). 240 patients (19.0%) were referred from different departments of the facility for better management, including rehabilitative support. Among the 1260 patients, degenerative peripheral joint disorders were the most common diagnosis (600, 46.0%), followed by inflammatory rheumatological conditions (300, 23.0%), non-infectious/non-inflammatory soft-tissue rheumatism (300, 23.0%), and radicular/referred LBP (100, 8.0%) (Fig. 1). These are discussed separately below.
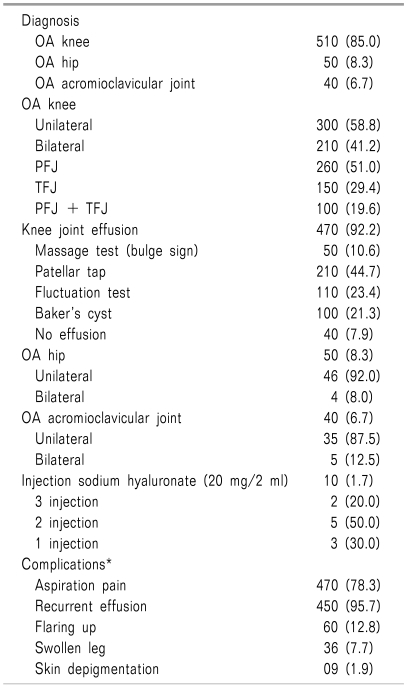
A total of 600 patients with different degenerative peripheral joint disorders (Table 2) were treated in the pain clinic. OA of the knee was the most frequent condition (510, 85%), followed by OA of the hip (50, 8.3%) and OA of the acromioclavicular joint (40, 6.7%). In cases of OA of the knee, unilateral presentation was seen in 300 cases and bilateral presentation was seen in 210 cases. The patello-femoral joint (PFJ) and tibio-femoral joint (TFJ) were involved in 260 and 150 cases, respectively. Both the PFJ and TFJ joints were involved in 100 cases (19.6%). The knee joint was deformed in 100 cases, and associated joint mouse or loose body was found in 29 patients. Presence of knee joint effusion was diagnosed by massage test, fluctuation test, and patellar tap in 50, 150, and 310 cases, respectively. In 40 patients there was no sign of effusion. A Baker's cyst was found to be present in 100 cases. Joint intervention was done with injection of methylprednisolone (40-80 mg) in OA of the knee and acromioclavicular joint, but not in the hip. Injection of sodium hyaluronate 20 mg/2ml was also given in 10 patients with OA of the knee refractory to I/A steroids. Out of these 10 patients, only 2 received the recommended dose (at least 3 injections in a single joint for 3 consecutive weeks). 5 patients received 2 and 3 patients received 1 injection. During and following joint procedures, complications were reported in 470 cases (78.3%), and the distribution was: aspiration pain (450, 95.7%), recurrent effusion (60, 12.8%), swollen leg (36, 7.7%), flare-ups (36, 7.7%), and skin depigmentation (9, 1.9%). Aspiration pain was common in the middle of the procedure. Recurrent effusion was common in deformed knee joints (58), with or without loose bodies in the joint. Flaring Flare-ups were common in OA of the knee treated with dry aspiration (27) during the first 24 hours following the procedure, and were managed with local ice application and diclofenac suppository (50 mg) when needed. Swollen leg following aspiration was mostly due to knee bandaging causing tight compression over popliteal vessels, resulting in a transient impairment of venous return and peripheral edema. There was no post-procedural joint infection.
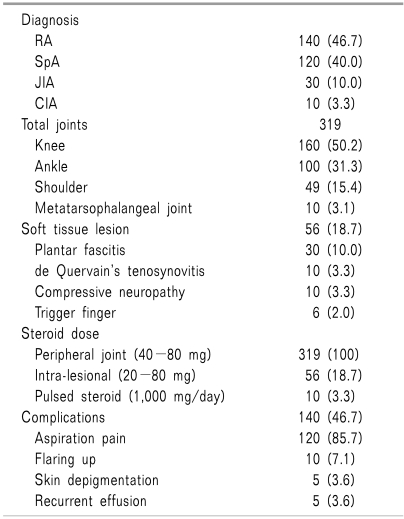
A total of 300 cases of different inflammatory rheumatological conditions (Table 3) were treated through intervention in different peripheral joints and soft tissues. Among these patients, the most frequent condition was RA (140, 46.7%), followed by SpA (120, 40.0%), juvenile idiopathic arthritis (30, 10.0%), and crystal induced arthritis (CIA) (10, 3.3%). Among patients with crystal arthritis, 8 cases were classified as gout and the other 2 were classified as pseudogout. As there were no facilities for crystal identification, synovitis was diagnosed on the basis of clinical examination only. Routine screening for serum uric acid and creatinine was also done in all suspected cases of CIA. Peripheral joint intervention was most common in the knee (160, 50.2%), followed by the ankle (100, 31.3%), shoulder (49, 15.4%), and 1st metatarsophalangeal joint (10, 3.1%). In addition to inflammatory peripheral joints, intervention was also done in inflammatory soft tissue, and commonly performed in plantar fascia (30, 10.0%), de Quervain's tenosynovial sheath (10, 3.3%), and trigger finger (6, 2.0%). All cases of plantar fasciitis were found in SpA variants. Trigger finger and de Quervain's tenosynovitis were classified as RA. Compressive neuropathy in the form of carpal tunnel syndrome (CTS) was also seen in 10 cases of RA. During and after intervention, common complications were joint aspiration pain (120, 85.7%), acute flare-up (10, 7.1%), skin depigmentation (5, 3.6%), and recurrent effusion (5, 3.6%). Aspiration pain was common in the knee joint at the mid point of the procedure. Pulsed steroids were given in 10 patients (3.3%) with RA or SpA during their acute flare-ups.
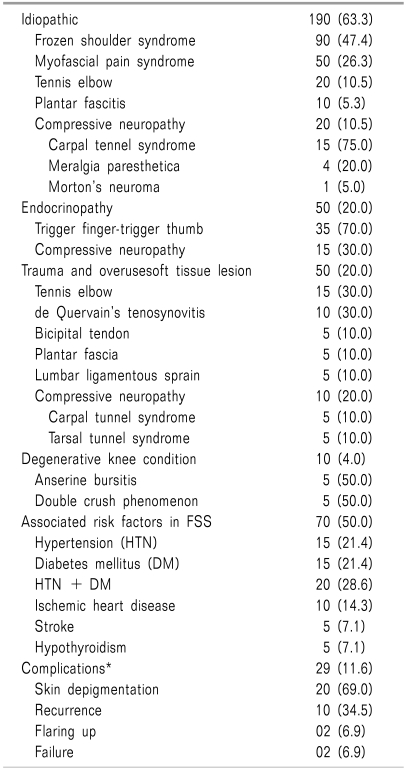
A total of 300 cases of STR (Table 4) were treated in this OPD, and are discussed under the following categories: idiopathic, endocrinopathy, OA of the knee, and trauma and overuse soft tissue lesion. Soft tissue lesion due to overuse and trauma was seen in 50 cases, and commonly involved the elbow (15, 30.0%), de Quervain's tenosynovial sheath (10, 20.0%), plantar fascia (5, 10.0%), bicipital tendon (5, 10.0%), and lower back, in which case the cause was lumbar ligamentous sprain (5, 10.0%). Soft tissue lesion of unknown etiology was found in 190 cases (63.3%), and common diagnoses were frozen shoulder (adhesive capsulitis) (90), MPS (50), tennis elbow (20), compressive neuropathy (20), and plantar fasciitis (10). MPS was most frequently found (27) in and around the scapulothoracic junction. Adhesive capsulitis was found with some co-morbid conditions, such as hypertension, DM, ischemic heart disease, stroke, and hypothyroidism. In endocrinopathy, STR was found in 50 cases, in the form of trigger finger/trigger thumb (35, 70.0%) and compressive neuropathy (15, 30.0%). Compressive neuropathy due to trauma/overuse was diagnosed in 10 patients (20.0%). Other than endocrinopathy and trauma/overuse, compressive neuropathy was also seen in the double crush phenomenon, and common diagnoses were CTS, tarsal tunnel syndrome (TTS), meralgia paresthetica, and Morton's neuroma. All 5 cases of TTS were found in lumbar spinal stenosis with a diagnosis of double crush phenomenon. Anserine bursitis was diagnosed in 5 cases of OA of the knee. Following IL steroid injection, reported complications were skin depigmentation (20, 69.0%), recurrence (10, 34.5%), acute flare-up (2, 6.9%), and failure (2, 6.9%). Skin depigmentation was common with procedures using triamcinolone in de Quervain's tenosynovial sheath.
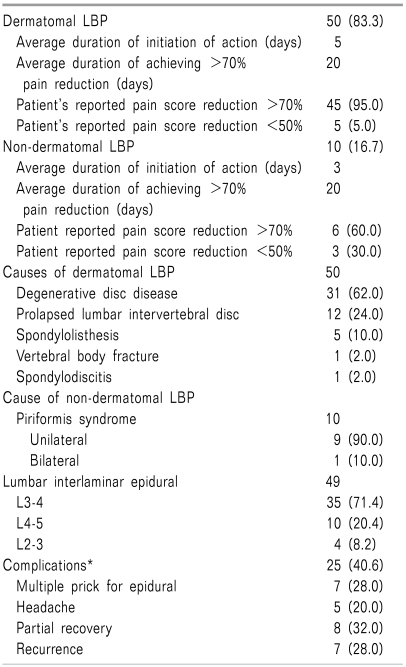
During the study period, a total of 100 patients with radicular and referred LBP were seen in the pain clinic. Out of these, 60 patients had radicular pain, and the remaining 40 had referred LBP. Sources of referred LBP were facet arthropathy, lumbar sacralization forming pseudoarthrosis with the sacrum, and sacroiliac arthropathy or dysfunction, diagnosed in 26, 9, and 5 cases, respectively. There was no intervention either in facet or sacroiliac joints. Radicular LBP (Table 5) was broadly categorized into two groups, dermatomal and non-dermatomal. A common presentation of dermatomal radiculopathy was lumbar spinal stenosis (50, 83.3%), due to degenerative disc disease (31, 62.0%), PLID (12, 24.0%), spondylolisthesis (5, 10.0%), spondylodiscitis (1, 2.0%), and vertebral body fracture (1, 2.0%). Among 50 cases of lumbar stenosis epidural steroid injection was given in 49 cases, no injection in spondylodiscitis. For dermatomal radiculopathy, lumbar epidural injections were given at the L3-4, L4-5, and L2-3 level in 35 (71.4%), 10 (20.4%), and 4 (8.2%) cases, respectively. As most of our patient could not understand VAS (visual analogue scale, 0-100 mm) for pain, a NRS (numerical rating scale, 0-100 mm) was used to report changes of pain after epidural injection where 0 indicates no pain and 100 indicates maximum pain. Before injection, all patients considered their pain as 100 mm (100%) and asked to rate their pain reduction by percentage after injection. At least 50% reduction of initial pain score within 3 weeks was defined as improvement. In our study subject, after epidural injection pain began to reduce at the end of the first week (mean 5 days). Out of 49, 45 patients reported 70% reduction of pain at third week of follow up (mean 20 days) and this improvement was maintained at least 6 months in 27 cases. On the other hand 70% pain reduction was sustained for mean 4 months in another 13 patients (26.0%), and all they needed a second dose of epidural steroid by this time. The patients' reported pain reduction score was below 50% in 4 cases of spondylolisthesis. Long-term data were not available in 4 patients of lumbar stenosis. Piriformis syndrome was the only cause of non-dermatomal radicular LBP, and was diagnosed in 10 patients (16.7%). Out of these, 6 patients gained 70% pain reduction after intervention, and this improvement was maintained for an average of 3 months with muscle relaxants (tolperison, cyclobenzaprine, or both). Common procedural and postprocedural complications were multiple pricks in lumbar epidurals (7, 28.0%), headache (5, 20.0%), partial recovery (8, 32.0%), and recurrence within 3 months (7, 28.0%).
Go to : 
Common inflammatory rheumatological conditions include SpA, RA, vasculitis, systemic lupus erythematosus, and mixed connective tissue disease, where along with spinal and peripheral joints, periarticular soft tissues also can be involved. Soft tissue lesions in the form of enthesitis are more common in SpA than in other inflammatory joint disorders, and are mostly distributed in the Achilles tendon and plantar fascia. It also can be seen in the head/base of the metatarsal bone, origin of the adductor muscle of the thigh, extensor tendon, anterior superior iliac spine, iliac crest, symphysis pubis, or de Quervain's tenosynovial sheath, etc. [17]. Compressive neuropathy due to soft tissue inflammation at the nerve tunnel is common in RA [18]. In inflammatory rheumatological conditions, spine and peripheral joints respond well to steroids/NSAIDs (non-steroidal anti-inflammatory drugs) and DMARDs (disease modifying anti-rheumatic drugs) [19,20]. Pulsed steroid (375-1,000 mg) in 5% dextrose aqua is a treatment option in acute flare-ups of RA/SpA, usually practiced for 3-5 consecutive days [21]. Osteoarthritis predominantly affects the weight bearing lower extremity joints and is more common in the knee than in the hip, ankle, or foot joints. In the upper extremity, OA usually involves the acromioclavicular joint. Along with oral medications, I/A steroids and local anesthetics can be effective in both inflammatory and degenerative joint disorders [20]. I/A injection of sodium hyaluronate (20 mg/2 ml) is a treatment option in OA of the knee responding poorly to available conservative approaches [22]. To reduce joint pain and repair damaged cartilage, at least 3 injections should be given in each joint for three successive weeks [22]. I/A ozone prolotherapy is also effective in knee OA, as described in some research articles [23,24]. After aspiration, bandaging can be done to prevent recurrent effusion, followed by restriction of knee weight bearing activities for the next 24-48 hours. Common complications during and after joint injection are aspiration pain, recurrent effusion, transient rise of blood pressure (steroid-induced), acute flare-up, injection site pain, overlying skin depigmentation, joint infection, or avascular necrosis. Aspiration pain is more common if joint procedures are done without local anesthesia. Acute flare-up is possible within the first 24-72 hours following the procedure, and generally resolves spontaneously, but can be managed with local application of ice/cold compresses. Recurrent effusion is more common in deformed joints. Overlying skin depigmentation has been reported following triamcinolone-mediated joint/soft tissue intervention [25]. In this recent study, a total of 300 cases of different inflammatory rheumatological conditions and 600 cases of degenerative peripheral joint disorders were treated in the pain clinic. In both of these conditions, the most common site of peripheral joint intervention was the knee. Soft tissue rheumatism (STR), soft tissue lesion, and compressive neuropathy were common in inflammatory rather than degenerative peripheral joint disorders. Inflammatory soft tissue lesions were commonly distributed in the plantar fascia (30), de Quervain's tenosynovial sheath (10), and trigger finger (6). Heel enthesitis (plantar fasciitis and Achilles tendinitis) was common in SpA. All compressive neuropathy in the form of CTS was classified as RA. I/A steroids were given to reduce joint pain, and the knee joint received a maximum dose (80 mg) of methylprednisolone among all peripheral joints. Injection of sodium hyaluronate 20 mg/2 ml was given in 10 cases of OA of the knee. Of these patients, only 2 received the recommended dose (at least 3 injections for 3 consecutive weeks). Pulsed steroids were given only in 10 cases (3.3%) of RA and SpA during acute flare-ups. To avoid development of avascular necrosis, we did not perform any steroidal intervention in the hip joint. During and following joint procedures, reported complications were aspiration pain, recurrent effusion, flare-ups, and skin depigmentation. Recurrent effusion was common in deformed knee joints (58). Flare-ups were documented within the first 24 hours. Aspiration pain was more common in the knee joint at the midpoint of the procedure, as we didn't use local anesthetic before joint injection. Skin depigmentation was found more frequently with triamcinolone acetonide preparation.
Non-infectious and non-inflammatory STR [26] has two basic patterns: compressive neuropathy and soft tissue lesion proper. Compressive neuropathy is commonly distributed in the carpal tunnel, tarsal tunnel, fibular neck (compression of the common peroneal nerve at the fibular neck), Guyon's canal (ulnar border of the wrist compressing the ulnar nerve) [27], anterior superior iliac spine (meralgia paresthetica), and interdigital nerve (Morton's nerve). Compressive neuropathy can also sometimes be associated with cervical and lumbar spinal stenosis, termed double crush phenomenon [27]. On the other hand, soft tissue lesion proper is usually distributed in the wrist/hand (de Quervain's tenosynovial sheath, flexor tendon), shoulder girdle (bicipital tendon, supraspinatus tendon), elbow (lateral and medial epicondyle), heel (plantar fascia, Achilles tendon), extensor tendon of hand/foot dorsum, knee (anserine bursa), and shoulder joint capsule [28, 29]. All of these soft tissues can be involved in different localized or systemic musculoskeletal disorders. In addition to inflammatory/degenerative rheumatological conditions, STR can also be found in endocrine disorders, overuse, and trauma, or it may be idiopathic [18,29,30]. Adhesive capsulitis is the leading presentation of idiopathic STR in some co-morbid conditions, such as DM, hypo- or hyperthyroidism, stroke, dyslipidemia, or ischemic heart disease. Patients usually complain of shoulder/arm pain with restricted movements impairing daily activities [27]. Other than adhesive capsulitis, MPS is another common pattern of idiopathic STR [30]. In overuse syndrome, it commonly involves the shoulder girdle (bicipital tendinitis, supraspinatus tendinitis), elbow (tennis and golfer's elbow), and wrist/hand (de Quervain's tenosynovitis, trigger finger/trigger thumb) [31-33]. All of these soft tissues can also be involved in DM, hypothyroidism, acromegaly, or postmenopausal women, due to associated hormonal imbalance [27,34]. Along with oral medications (NSAIDs, analgesic or neuropathic agents), physical interventions (therapeutic pulsed ultrasound), therapeutic exercise, and IL steroid/ozone or prolotherapy can also be effective in managing this, a common rheumatological manifestation. When all of these approaches have failed, then surgery can be done to release soft tissues [27,28]. Following local steroid injection, common complications are skin depigmentation, soft tissue injury, injection site pain, flare-ups, and infection. Rupture of the plantar fascia is also possible after local steroid injection. In our study, idiopathic soft tissue lesions were treated in 190 cases (63.3%), and the most common presentation was adhesive capsulitis (90). MPS was diagnosed in 50 cases. Soft tissue lesions due to overuse and blunt trauma were seen in 50 cases, and most common (15) in the elbow region. In the lower back, pain was due to lumbar ligamentous sprain and MPS. Anserine bursitis was diagnosed in 5 cases of OA of the knee. STR in the form of trigger finger/trigger thumb (35) and compressive neuropathy (15) was diagnosed in 50 patients having endocrine problems. Traumatic compressive neuropathy was seen in 10 cases. Other than trauma/overuse or endocrinopathy, compressive neuropathy can also be seen in cervical and lumbar spondylosis, and in our recent study all 5 cases of TTS were found in lumbar spinal stenosis, with a final diagnosis of double crush phenomenon. Idiopathic compressive neuropathy was also seen in 20 patients. Following IL steroid injection, reported complications were skin depigmentation, recurrence, acute flare-up, and failure. Skin depigmentation was seen in 20 cases (69.0%) following triamcinolone injection.
The basic patterns of LBP are localized, radicular, and referred. Localized and radicular LBP occur due to spine degeneration, inflammation, infection, trauma, tumor, or disc herniation. Radiculopathy can be both dermatomal and non-dermatomal; dermatomal pain follows the corresponding nerve root, whereas in non-dermatomal radiculopathy, the pain is due to involvement of multiple nerve roots or a single nerve, and doesn't follow a single nerve root [35]. One of the common but rarely considered causes of non-dermatomal radicular LBP is piriformis syndrome, which may develop following low back trauma, and in which the patient may feel discomfort in sitting for long periods, standing, walking, and even in forward bending [36]. Along with NSAIDs, muscle relaxants, and neuropathic agents, radiculopathy can be treated with physical interventions (therapeutic ultrasound, transcutaneous electrical nerve stimulation) and therapeutic exercises [37]. For radicular lower back pain, epidural injections using methylprednisolone and local anesthetics may be helpful. Lumbar epidural injections can be performed using anatomical landmarks (LOR, loss of resistance), and also with C-arm fluoroscopy, and are commonly performed at the L3-4/L4-5 level [14, 15]. This treatment is effective in spinal stenosis, PLID, and degenerative disc disease, but less effective in spondylolisthesis [38,39]. On the other hand, in piriformis syndrome, respective muscle intervention can be done with steroids and local anesthetics using anatomical landmarks, motor stimulation, C-arm fluoroscopy, and ultrasound guidance [40]. After steroidal intervention, recurrence of piriformis muscle pain is common, requiring botulinum toxin injection [41]. Piriformis injection followed by piriformis muscle stretching exercises may provide the maximum benefit. Referred LBP is mostly due to sacroiliac and facet arthropathy, but it can also be viscerogenic in source. Facet and sacroiliac arthropathy can be treated with I/A steroid injections or radiofrequency ablation of the respective joint/nerve supplying the joint [42,43]. Radiculopathy due to disc prolapse not responding to epidural steroids can be managed with ozone nucleolysis [44]. IDET is a good option for internal disc disruption, and non-traumatic vertebral body fracture can be managed with vertebroplasty [45,46]. During and after epidural/facet joint injection, common complications are headache, neurogenic shock, thecal sac puncture, temporary dizziness, multiple pricks, vasovagal reaction, infection, potential weight gain, transient rise of blood pressure/hyperglycemia (steroid-induced), and failure, among others [47,48]. In the current study, a total of 60 patients with radicular LBP were treated in the pain clinic by IPM. A common presentation of dermatomal radiculopathy was lumbar spinal stenosis (50, 83.3%), due to degenerative disc disease, PLID, spondylolisthesis, spondylodiscitis, and vertebral body fracture. All cases of dermatomal radiculopathy except only one infectious lumbar spondylodiscitis were treated with lumbar epidural injection using the LOR technique. The majority of lumbar interlaminar epidural injections were performed at L3-4 (35, 71.4%). It takes about 1 week (mean 5 days) to initiate pain reduction, and a patients' reported 70% reduction of pain was sustained in 27 patients even after 6 months following the intervention. This pain reduction was sustained for only 4 months in another 13 patients, with a repeated epidural injection necessary by this time. Numerical pain reduction score was below 50% in 4 cases of spondylolisthesis. In piriformis syndrome, the average duration between intervention and at least 50% pain improvement was 5 days. Out of 10 patients, 6 obtained 70% reported pain reduction, which was maintained for an average of 3 months with NSAIDs (diclofenac sodium) and muscle relaxants (tolperison and/or cyclobenzaprine). Common post-procedural complications were multiple pricks in lumbar epidural injections, headache, flare-ups, and partial recovery.
It can be concluded that IPM is gaining popularity throughout the world in the management of painful musculoskeletal conditions. Along with anesthesiologists, physiatrists have a definitive role in IPM. To better serve pain sufferers, the physiatrist must work hand-in-hand with other specialties. In this article, we describe a few procedures performed in the PMR department of a tertiary medical college hospital in Bangladesh using only anatomical landmarks, so accuracy was not ensured. We did not perform any intervention in a facet joint, sacroiliac joint, sympathetic ganglion, vertebral body, or intervertebral disc using radiofrequency ablation, IDET, ozone nucleolysis, or steroid injection because of: (1) Lack of a C-arm facility in the PMR department; (2) Greater reliance on oral medications; (3) Lack of knowledge of diagnostic musculoskeletal ultrasonogram or fluoroscopy; (4) Lack of exposure to interventional pain procedures; and (5) Poor knowledge about the spectrum of IPM. Therefore, we recommend: (1) Development of an IPM unit in the PMR department; (2) Greater emphasis on IPM as a separate entity; (3) Increased collaboration with other institutes/departments dealing with interventional procedures both at home and abroad; (4) Development of skills in IPM through special training; and (5) Training in diagnostic musculoskeletal ultrasonogram/electrodiagnosis to ensure a safe and accurate interventional approach, with an ultimate goal of serving pain sufferers to ensure them a better quality of life.
Go to : 
ACKNOWLEDGEMENTS
We thank the staff of the Physical Medicine and Rehabilitation Department of Chittagong Medical College Hospital for their help in data collection from hospital records. We also acknowledge the people who work in the computer and printing department of Feni Diabetes Hospital (FDH) for their continuous support in preparation of the manuscript. Our special thank to the Opsonin pharmaceutical (Bangladesh) limited because of their support providing necessary documents required for manuscript preparation.
Go to : 
References
1. Santhos A, Thomas DO, Walsh MC. Interventional pain management: a comprehensive approach to chronic pain. 2010. 1. 02. 2011 Feb 23. Available at: http://www.spineuniverse.com.
2. Johnson EW. The interventional physiatrist: a new subspecialist has been born! Am J Phys Med Rehabil. 2001; 80:1–3.

3. Carrino JA, Morrison WB, Parker L, Schweitzer ME, Levin DC, Sunshine JH. Spinal injection procedures: volume, provider distribution, and reimbursement in the US medicare population from 1993 to 1999. Radiology. 2002; 225:723–729. PMID: 12461252.

4. Boswell MV, Shah RV, Everett CR, Sehgal N, McKenzie Brown AM, Abdi S, et al. Interventional techniques in the management of chronic spinal pain: evidence-based practice guidelines. Pain Physician. 2005; 8:1–47. PMID: 16850041.
5. Drusso. Interventional PM&R. 2002. 7. 07. 2011 Apr 5. Available at: http://forums.studentdoctor.net/archive/index.php/t-40131.html.
6. Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991; 34:1218–1227. PMID: 1930310.

7. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27:361–368. PMID: 6231933.
8. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324. PMID: 3358796.

9. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Classification of osteoarthritis of the knee Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986; 29:1039–1049. PMID: 3741515.

10. Solomon L, Warwick D, Nayagam S. Apley's system of orthopaedics and fractures. 2001. 8th ed. New York: Oxford University Press;p. 399–400.
11. Sinaki M, Mokri B. Braddom RL, editor. Low back pain and disorders of the lumbar spine. Randall's textbook of physical medicine and rehabilitation. 1996. Philadelphia: WB Saunders;p. 813–850.
12. Canoso JJ. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Aspiration and injection of joints and periarticular tissues. Practical rheumatology. 2004. Philadelphia: Mosby Elsevier;p. 73–84.
13. Kreder HJ. Fam AG, Lawry GV, Kreder HJ, editors. The knee. Musculoskeletal examination and joint injections techniques. 2006. Philadelphia: Mosby Elsevier;p. 83–84.

14. Abram SE. Treatment of lumbosacral radiculopathy with epidural steroids. Anesthesiology. 1999; 91:1937–1941. PMID: 10598637.

15. Weinstein SM, Herring SA. NASS. Lumbar epidural steroid injections. Spine J. 2003; 3(3 Suppl):37S–44S. PMID: 14589216.

16. Gonzalez P, Pepper M, Sullivan W, Akuthota V. Confirmation of needle placement within the piriformis muscle of a cadaveric specimen using anatomic landmarks and fluoroscopic guidance. Pain Physician. 2008; 11:327–331. PMID: 18523503.
17. Burgos-Vargas R. Weisman MH, van der Heijde D, Reveille JD, editors. The juvenile-onset spondyloarthritides. Ankylosing spondylitis and the spondyloarthropathies. 2006. Philadelphia: Mosby Elsevier;p. 96–97.

18. Chamberlain MA, Corbett M. Carpal tunnel syndrome in early rheumatoid arthritis. Ann Rheum Dis. 1970; 29:149–152. PMID: 5427410.

19. Dougados M, Gueguen A, Nakache JP, Velicitat P, Veys EM, Zeidler H, et al. Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology (Oxford). 1999; 38:235–244. PMID: 10325662.

20. Buttgereit F, Straub RH, Wehling M, Burmester GR. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum. 2004; 50:3408–3417. PMID: 15529366.

21. Peters ND, Ejstrup L. Intravenous methylprednisolone pulse therapy in ankylosing spondylitis. Scand J Rheumatol. 1992; 21:134–138. PMID: 1604251.

22. Arramon JY, Bergamasco P, Blasco G, Vincent P. Intraarticular sodium hyaluronate (Arthrum(R)H 2.0%) in the treatment of patients with knee osteoarthritis: a multi-centric, randomized, comparative clinical trial. Minerva Ortopedicae e Traumatologica. 2008; 59:69–79.
23. Al-Jaziri AA, Mahmoodi SM. Painkilling effect of ozoneoxygen injection on spine and joint osteoarthritis. Saudi Med J. 2008; 29:553–557. PMID: 18382798.
24. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000; 6:68–74. 77–80. PMID: 10710805.
25. Fam AG. Fam AG, Lawry GV, Kreder HJ, editors. Principles of joint and periarticular aspirations and injections. Musculoskeletal examination and joint injections techniques. 2006. Philadelphia: Mosby Elsevier;p. 128–131.

26. Hudson N, Fitzcharles MA, Cohen M, Starr MR, Esdaile JM. The association of soft-tissue rheumatism and hypermobility. Br J Rheumatol. 1998; 37:382–386. PMID: 9619887.

27. Solomon L, Warwick D, Nayagam S. Apley's system of orthopaedics and fractures. 2001. 8th ed. New York: Oxford University Press;p. 247–253.
28. Dalton SE. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. The shoulder. Practical rheumatology. 2004. 3rd ed. Philadelphia: Mosby Elsevier;p. 198–199.
29. Reveille JD. Soft-tissue rheumatism: diagnosis and treatment. Am J Med. 1997; 102:23S–29S. PMID: 9217556.

30. Smythe H. Links between fibromyalgia and myofascial pain syndromes. J Rheumatol. 1992; 19:842–843. PMID: 1404117.
31. Thompson JS, Phelps TH. Repetitive strain injuries. How to deal with 'the epidemic of the 1990s'. Postgrad Med. 1990; 88:143–149. PMID: 2243822.
32. Kivi P. The etiology and conservative treatment of humeral epicondylitis. Scand J Rehabil Med. 1983; 15:37–41. PMID: 6828831.
33. Clarke MT, Lyall HA, Grant JW, Matthewson MH. The histopathology of de Quervain's disease. J Hand Surg Br. 1998; 23:732–734. PMID: 9888670.

34. Yosipovitch G, Yosipovitch Z, Karp M, Mukamel M. Trigger finger in young patients with insulin dependent diabetes. J Rheumatol. 1990; 17:951–952. PMID: 2213763.
35. Borenstein DG. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Low back pain and lumbar spinal stenosis. Practical rheumatology. 2004. 3rd ed. Philadelphia: Mosby Elsevier;p. 178.
36. Jeon SY, Moon HS, Han YJ, Sung CH. Post-radiation piriformis syndrome in a cervical cancer patient -a case report-. Korean J Pain. 2010; 23:88–91. PMID: 20552082.

37. Boyajian-O'Neill LA, McClain RL, Coleman MK, Thomas PP. Diagnosis and management of piriformis syndrome: an osteopathic approach. J Am Osteopath Assoc. 2008; 108:657–664. PMID: 19011229.
38. Kaplan M, Derby R. Epidural corticosteroid injections: When, why, and how. J Musculoskel Med. 1998; 15:39–46.
39. Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007; 356:2257–2270. PMID: 17538085.

40. Byeon GJ, Kim KH. Piriformis syndrome in knee osteoarthritis patients after wearing rocker bottom shoes. Korean J Pain. 2011; 24:93–99. PMID: 21716617.

41. Fishman LM, Anderson C, Rosner B. BOTOX and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil. 2002; 81:936–942. PMID: 12447093.

42. van Kleef M, Barendse GA, Kessels A, Voets HM, Weber WE, de Lange S. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976). 1999; 24:1937–1942. PMID: 10515020.

43. Slipman CW, Bhat AL, Gilchrist RV, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003; 3:310–316. PMID: 14589192.

44. Muto M, Andreula C, Leonardi M. Treatment of herniated lumbar disc by intradiscal and intraforaminal oxygen-ozone (O2-O3) injection. J Neuroradiol. 2004; 31:183–189. PMID: 15356443.

45. Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976). 2000; 25:923–928. PMID: 10767803.

46. Saal JA, Saal JS. Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Clin Sports Med. 2002; 21:167–187. PMID: 11877870.

47. Botwin KP, Gruber RD, Bouchlas CG, Torres-Ramos FM, Freeman TL, Slaten WK. Complications of fluoroscopically guided transforaminal lumbar epidural injections. Arch Phys Med Rehabil. 2000; 81:1045–1050. PMID: 10943753.

48. Bakshi S, Abrahamsen GW. Baheti DK, Bakshi S, Gupta S, Gehdoo RP, editors. Lumbar facet and median branch block. Interventional pain management a practical approach. 2009. New Dehli: Jaypee Brothers Medical Publishers Ltd;p. 196.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download